Matter ICSE Class-6th Concise Selina Chemistry Solutions Chapter-3. We Provide Step by Step Answer of Objective, True False , Fill in the blanks , Match the following , Short/Long Answer Type of Exercise-3 Matter . Visit official Website CISCE for detail information about ICSE Board Class-6.
Matter ICSE Class-6th Concise Selina Chemistry Solutions Chapter-3
EXERCISE- I Chapter- 3 Matter ICSE Class-6th Concise
Question 1.
Define matter.
Answer:
Anything that has mass and occupies space is called matter.
Question 2.
What are the two main types of matter? Give two examples for each type.
Answer:
The two main types of matter are :
- Living matter: The earth is home to all kinds of plants and animals. They can grow, move and reproduce on their own. Examples : Plant, lotus, animals, human etc.
- Non-living matter : Most of the matter in the universe is non-living. It means that it does not grow, move or reproduce on its own. It can be natural or man made.
(a) Natural matter: It occurs in nature and can be used to make more useful substances, e.g., wood, coal, silk, water, stone, cotton, jute, cereals, fruits, etc.
(b) Man-made matter : It is produce artificially from natural matter, e.g., plastics, soaps, detergents, medicines, glass, nylon, steel, ceramic, etc.
Question 3.
Differentiate between living and non-living matter.
Answer:
Living matter:
- The earth is home to all kinds of plants and animals. They can grow, move and reproduce on their own.
- It is natural only.
Non-living matter:
- Most of the matter in the universe is non-living. It means that it does not grow, move or reproduce on its own.
- It can be natural or man made.
Question 4.
Select natural and man made matter from the following
list: Wood, plastic, silk, medicines, detergents, coal, water, ceramic, cotton, glass, nylon, fruits.
Answer:
Natural matter: Wood, silk, coal, water, fruits.
Man made matter: Plastic, medicines, detergents, ceramic, cotton, glass, nylon.
EXERCISE-II Matter ICSE Class-6th Concise
Question 1.
Name the smallest particle from which matter is made up.
Answer:
The smallest particle from which matter is made up is atom.
Question 2.
What are molecules ?
Answer:
Molecules are the smallest unit of matter. They exhibit all the properties of that kind of matter and is capable of independent existence.
Question 3.
Give one difference between atoms and molecules.
Answer:
Atoms may or may not have independent existance. While molecules have independent existence.
Question 4.
Define:
(a) Intermodular force of attraction.
(b) Intermodular space.
Answer:
(a) The molecules of matter are always in motion and attract each other with a force, and this force is called intermodular force of attraction due to which they are held together.
(b) The molecules can move only when there are gaps or space between them, this space is called inter modular space.
Question 5. Matter ICSE Class-6th Concise
Name the three states of matter and define them.
Answer:
The three states of matter are :
- Solid State
- Liquids
- Gases :
Solid State : The molecules are very close to each other hence inter modular spaces are small and inter modular force is strong.
Hence solids have definite volume, rigid, retain definite shape and are in compressible.
Liquids : The molecules are less closely packed have more inter molecular spaces than solid, less stronger forces than solids.
Hence liquids have definite volume but no definite shape. They take the shape of container in which they are put.
Gases : The molecules in the gases are far apart with weakest force of attraction. Hence gases have neither definite volume nor definite shape but easily compressible.
Question 6.
What are fluids ? Give two examples
Answer:
Substances that can flow are called fluids. Both gases and liquids are fluids, e.g. gases (carbon dioxide, hydrogen), liquids (water, petrol and acid).
Question 7.
Classify the following into solids, liquids and gases.
Oxygen, milk, common salt, wax, stone, L.P.G, carbon- dioxide, sugar, mercury, coal, blood, butter, copper, coconut oil, kerosene.
Answer:

Question 8.
Give reasons
(a) Liquids and gases flow but solids do not ?
(b) A gas Alls up the space available to it.
(c) The odour of scent spreads in a room.
(d) We can walk through air.
(e) Liquids have definite volume but no definite shape.
(f) When a teaspoon of sugar is added to half a glass of water and stirred, the water level in the glass remains unchanged.
(g) When an empty gas jar is inverted over a gas jar containing a coloured gas, the gas also spreads into the empty jar.
(h) A red ink drop added to small amount of water in a glass turns the water red in some time.
Answer:
(a) The molecules of liquids and gases are far apart i.e. have more gaps, intermolecular attraction force is very less as compared to solids, hence liquids and gases can flow but solids do not as gaps*in solid molecules is less and molecular force of attraction very strong.
(b) Intermolecular force of attraction is least and intermolecular spaces are very large, hence gases can fill up the space available to them.
(c) Scent fumes (molecules) being gases fill the spaces between air molecules and the molecules of air fill the spaces between scent molecules due to diffusion, fumes spread into a room.
OR
Due to inter-mixing of scent molecules and air molecules, scent fumes spread into the room.
(d) The molecules of air are far apart i.e. large gaps and we can walk through air easily.
(e) The molecules of liquid are loosely packed and intermolecular force of attraction is small but number of molecules in it remain the same. Hence liquids have definite volume but no definite shape.
(f) When a teaspoon of sugar is added to half a glass of water and stirred, the water level in the glass remains unchanged because the sugar particles are adjusted between the water molecules as inter-molecular gaps are more in liquids.
(g) This is because Gases can diffuse or flow in all directions.
(h) When we put a drop of red ink in a glass of water, its particles diffuse with particles of water slowly but continuously and the water turns red.
Question 9. Matter ICSE Class-6th Concise
Define:
(a) cohesive force
(b) diffusion
(c) Brownian movement
Answer:
(a) Cohesive force : The force of attraction between particles of the same substance is called cohesive force.
(b) Diffusion: The phenomenon of intermixing of particles of one kind with another kind is called diffusion.
(c) Brownian movement: The zig-zag motion of particles suspended in a medium is called Brownian movement
Question 10.
Why is an egg kicked out of a bottle when air is blown inside the bottle?
Answer:
When we invert the bottle and blow air into the bottle throw the side opening. It creates high pressure inside the bottles and the egg is kicked out of the bottle.
EXERCISE-III Matter ICSE Class-6th Concise
Question 1.
State the three effects of heat on matter.
Answer:
When a substance is heated, it can cause.
- Interconversion of states of matter.
- Thermal expansion of the substance.
- Chemical change.
Question 2.
(a) Define : interconversion of states of matter.
(b) What are the two conditions for the interconversion of states of matter ?
Answer:
(a) The process by which matter changes from one state to another and back to original state, without any change in its chemical composition.
(b) Two conditions are :
- Change in temperature
- By applying pressure
Question 3.
Define the following terms:
(a) Fusion
(b) Vaporisation
(c) Condensation
(d) Sublimation
(e) Diffusion
(f) Melting point
(g) Boiling point
(h) Liquefaction
Answer:
(a) Fusion : The heating process by which a solid changes into the liquid state is called fusion.
(b) Vaporisation : The heating process by which a liquid changes into its vapour state is called vaporisation.
(c) Condensation : The process by which a substance in gaseous state changes into its liquid state is called condensation.
(d) Sublimation: The change of solid on heating to vapours directly and vice-versa without passing through the liquid state is called sublimation.
(e) Diffusion : The phenomenon of intermixing or spreading of gaseous molecules is called diffusion.
(f) Melting point: The fixed temperature at which a solid changes into a liquid at a given pressure is called its melting point. The temperature remains constant as long as the conversion is going on.
(g) Boiling point: The fixed temperature at which a liquid starts changing into gaseous state is called its boiling point. The temperature remains constant till the whole of the liquid changes into gaseous state.
(h) Liquefaction : Change of vapours on cooling to liquid is called liquefaction
Question 4.
Differentiate between:
(a) Solidification and condensation
(b) Melting and boiling
(c) Gas and vapour
(d) Miscible and immiscible liquids.
Answer:
(a) Solidification : The process of changing liquid into a solid state by cooling is known as solidification.
Example : water → ice.
Condensation : The process of changing a gas or vapour state to a liquid state by cooling is known as condensation. Example : steam → water.
(b) Melting : The fixed temperature at which a solid changes into a liquid at a given pressure is called its melting point.
e.g. ice → water.
Boiling : The fixed temperature at which a liquid starts changing into gaseous state is called its boiling point.
e.g. water → steam.
(c) Vapourisation : The process by which a substance changes from a liquid state to vapour state is called vaporisation or evaporation.
e.g., Water changes into gaseous state on heating.
Gas : The substance which remain in the gaseous state under normal conditions of temperature and pressure are called gases.
e.g, Oxygen, hydrogen, nitrogen.
(d) Miscible: Liquids which mix with each other are called miscible liquids. Example : Water and alcohol.
Immiscible liquids : Liquids which do not mix with each other are called immiscible liquids. Example Water and oil.
Question 5.
Give reasons :
(a) How is interconversion of states of matter different from chemical reaction ?
(b) Why a solid does not flow, but a liquid flows ?
Answer:
(a) During interconversion of state of matter composition
of substance remains the same matter changes from one state to another and back to the original state, while chemical reaction involves re-arrangement of the molecular structure and composition changes.
(b) In solids there is a strong force of attraction between the molecules and the space between them is very negligible. The molecules are therefore, not free to move. They merely vibrate about their mean positions. But in the case of liquids, the molecules are not very closely packed. They do not attract each other as strongly as the molecules of solids. Thus, the intermolecular spaces are larger and the molecules are able to move about more freely. This makes a liquid flow.
Question 6.
How does a liquid changes into its gaseous state? Explain ?
Answer:
As a liquid is heated, its particles starts gaining energy and move more vigorously which increases the gaps between the particles and decreasing the force of attraction. Ultimately a liquid changes into gaseous state.
Question 7.
Water cycle is an example of interconversion of states of water. Explain.
Answer:
Water from oceans, rivers, lakes from leaves of trees (transpiration) changes into vapours when temperature increases or evaporates and enters the atomsphere as clouds when temperature falls the vapours change into water and some of it in the form of snow fall on mountains and earth in the form of water and hales and this continues. Thus water cycle is example of interconvertion of states of water.
Question 8.
What happens to a metal ball when it is heated? What does this show?
Answer:
When metal ball is heated, it expands. This can be proved by following experiment:
Take a metallic ring and ball. Try to pass the metal ball through the ring. The ball is able to pass through the ring. Now heat the metal ball for 5-6 minutes. The hot ball is not be able to pass through the ring.
This shows that a solid expands on heating. Now cool the ball, it again passes through the ring. This shows that a solid contacts on cooling.
Question 9.
Why does a candle become smaller on burning with time?
Answer:
On heating, candle wax melts, then turns into vapour which reacts with air to produce two new substances, carbondioxide and water.
Therefore a candle on burning becomes smaller and smaller and the part of wax which has undergone chemical change cannot be recovered.
OBJECTIVE TYPE QUESTIONS Matter ICSE Class-6th Concise
Question 1.
Fill in the blanks :
(a) Water is a matter because it has ………… and occupies ………..
Water is a matter because it has mass and occupies space.
(b) Any matter which has a definite …… but no definite shape is called a …….
Any matter which has a definite volume but no definite shape is called a liquid
(c) ………. and ………….can flow.
Liquids and gases can flow
(d) The molecules are at a greater distance in …………….. compared to liquids.
The molecules are at a greater distance in gases compared to liquids.
(e) Water boils at 100 °C.
(f) The physical state of a substance, which has neither fixed volume nor fixed shape is a………..
The physical state of a substance, which has neither fixed volume nor fixed shape is a gas.
Question 2.
Write whether the following statements are true or false.
(a) Only water can exist in three different states.
(b) If the container in which a gas is collected has an opening, the gas will flow out and spread itself indefinitely.
(c) Solids have the larg^* inter-molecular space.
(d) There is no difference between evaporation and boiling.
(e) All solids, on heating, first change to the liquid and then to the gaseous state always.
(f) The intermolecular force of attraction is the weakest in gases.
(g) A gas has no free surface.
Answer:
(a) True
(b) True
(c) False
Correct: Solids have the very small (negligible) inter-molecular space.
(d) False
Correct : There is a difference between evaporation and boiling.
(e) False
Correct : Few solids, on heating, first change to the liquid and then to the gaseous state always.
(f) True
(g) True
Question 3.
For each of the following statements, say whether it describes a solid, a liquid or a gas.
(a) Particles move about very quickly but do not leave the surface : Liquid
(b) Particles are quite close together : Solid
(c) Particles are far apart and move in all directions : Gas
Question 4. Matter ICSE Class-6th Concise
Match the following :
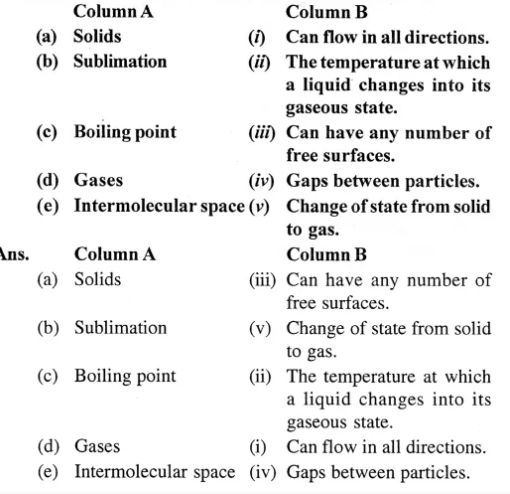
Question 5.
Name the phenomenon which causes the following changes:
(a) Formation of water vapour from water.
(b) Disappearance of camphor when exposed to air.
(c) Conversion of ice into water.
(d) Conversion of water into steam.
Answer:
(a) Formation of water vapour from water is vaporisation.
(b) Disappearance of camphor is sublimation.
(c) Conversion of ice into water is melting.
(d) Conversion of water into steam is boiling.
Question 6.
Give two examples for each of the following :
(a) Substances which sublime.
(b) Substances which do not change their states.
(c) Substances which are rigid and not compressible.
Answer:
(a) Naphthalene, camphor, dry ice.
(b) Oxygen, hydrogen, nitrogen
(c) Glass, stone, pen.
MULTIPLE CHOICE QUESTIONS Matter ICSE Class-6th Concise
1. Which one is a kind of matter :
(a) light
(b) petroleum
(c) sound
(d) heat
Answer:
petroleum
2. the state of matter which has no definite shape or volume is called
(a) solid
(b) liquid
(c) gass
(d) water
Answer:
gass
3. There are large intermolecular gaps in
(a) water
(b) iron ball
(c) common salt ..
(d) air
Answer:
air
4. All kinds of matter
(a) occupy space and have a definite mass
(b) have mass and a definite shape
(c) can change their states
(d) have a definite volume
Answer:
occupy space and have a definite mass
5. A kind of matter which can sublime is
(a) water
(b) plastic
(c) milk
(d) iodine
Answer:
iodine
6. A substance which can change its state
(a) wood
(b) oxygen
(c) paper
(d) cloth
Answer:
oxygen
7. The process by which a solid changes into a liquid is called
(a) freezing
(b) melting
(c) condensation
(d) evaporation
Answer:
melting
PROJECT Matter ICSE Class-6th Concise
Question 1.
Fill the following chart showing twelve solids, twelve liquids, four gases and eight materials.
Answer:
Solids : (1) Mobile. (2) Pen. (3) Pair of shoes. (4) A T.V. set (5) Chair. (6) Telephone. (7) Remote control. (8) Wood. (9) Ornaments. (10) Scissors. (11) Eraser. (12) Mirror.
Liquids : (1) Ink. (2) Water. (3) Lemon juice. (4) Cough syrup. (5) Mouth wash. (6) Petrol. (7) Kerosene oil. (8) Spirit. (9) Thinner. (10) Mercury. (11) Milk. (12) Copper sulphate solution.
Gases : (1) Hydrogen. (2) Oxygen. (3) Sulphur dioxide. (4) Chlorine gas.
Materials : (1) Paper. (2) Wood. (3) Iron nails. (4) Cement. (5) Tiles. (6) Plaster of paris. (6) Sand. (7) Iron rods. (8) Bricks.
Question 2.
Think and try to find a way to demonstrate water cycle in class.
Answer:
Do it yourself.
Question 3.
To identify materials of common use
Procedure – Just move around in your house – in the drawing room, sitting room, bedroom, kitchen, bathroom etc.
Identify the things and All in the blanks in the table given below:
- Study room
- Drawing room
- Kitchen
- bathroom
- another place
| S.No. Place | Name of the thing | Material used for making the thing |
|
||
Answer:
| S.No. Place | Name of the thing | Material used for making the thing |
| 1. Study room | Table, chair, books pens, pencils, table lamp, computer, fan | Wood, plastic, paper, glass, bakelite, connecting wires made of metal and plastics |
| 2. Drawing room | Sofa, table, chairs, fan, curtains, windows etc. | Wood, cloth, coir, paint, plastics, aluminium, glass etc. |
| 3. Kitchen | Taps, wash basin, cupboards, utencils, heating vessels strainer, containers aqua pure etc. | Steel, aluminium, glass, china clay, iron, plastic, copper, stainless steel, brass etc. |
| 4. Bathroom | Taps, tubs, mugs, tooth brush, tooth paste, soap, pail etc. | Iron, brass, stainless steel, plastics etc. |
| 5. Any other place | Scooter, car, road, rooms, trucks, shops. | Iron, rubber, coaltar, bricks, wood, plastics, clothes. |
Return To ICSE Class – 6 Concise Selina Chemistry Solution
Thanks



Dear Selina publishers,
We aren’t finding any option to download the notes, nor can copy or share them. It’s a donkey work for us when everything is available,school still wants teachers to type answer to all the lesson exercise notes and worksheets. Please can you make it available as a word file for Gr. ^ to 8 all Chemistry solutions?
Atleast make it available to copy paste the answers in word.
Looking forward for your reply.
regards,
Smita K
it is read only u can not copy or download.
content is available in read only format
for downloading we need it in pdf format but then we can not edit time to time for latest version
we are not selina publishers
our team working for helping ICSE and ISC student in absolutely free