Metallurgy ICSE Class-10 Concise Chemistry Selina Solution Chapter-7. We Provide Step by Step Answer of Exe-7(A) , Exe-7(B) , Exe-7(C) , Exe-7 (D) , Misc and Previous Year Questions of Exercise-7 Metallurgy ICSE Class-10 . Visit official Website CISCE for detail information about ICSE Board Class-10.
| Board | ICSE |
| Publications | Selina Publishers PVT LTD |
| Subject | Concise Chemistry |
| Class | 10th |
| writer | Dr SP Singh |
| Chapter-7 | Metallurgy |
| Topics | Solutions of Exe-7(A) , Exe-7(B) , Exe-7(C) , Exe-7 (D) , Misc and Previous Year Questions |
| Edition | 2021-2022 |
Metallurgy ICSE Class-10 Concise Chemistry Selina Solution Chapter-7
-: Select Topics :-
Exe-7(A) ,
Exe-7(B) ,
Exe-7(C) ,
Misc ,
How to solve Metallurgy in Chemistry for ICSE Class 10
Read the Chapter -7 Metallurgy carefully. Focus on reaction involve during Calcination and Roasting. Various Step involve in extraction in Metallurgy. Obtaining Aluminium from their Ore. For more practice on Metallurgy try to solve exercise of other publications such as Goyal Brothers Prakshan / new Simplified Dr Dalal Chemistry.
Selina Concise Solution Metallurgy ICSE Chemistry Exercise – 7(A)
Page-123
Question 1
(a) Name the three classes in which elements are classified. Which was the first metal used by man?
(b) Name the metal and non-metal present in abundance in the earth crust.
Answer 1
(a) Three classes in which elements are classified are:
Metals, Non-metals and Metalloids
Question 2
Name the metal which is a constituent of: (a) Blood pigment (b) Plant pigment
Answer 2
(a) The metal which is a constituent of blood pigment is Iron (Fe)
(b) The metal which is a constituent of plant pigment is Magnesium (Mg).
Question 3
Give the importance of the following in living beings:
(a) Nitrogen, (b) Hydrogen, (c) Carbon
Answer 3
(a) Nitrogen: It is used to preserve food.
(b) Hydrogen: It is used in the hydrogenation of vegetable oils to make ghee.
(c) Carbon: It is essential for the growth and development of living beings.
Question 4
State the position of the following in the periodic table:
(a) Alkali metals (b) Alkaline earth metal (c) Halogens (d) Aluminium
Answer 4
(a) Alkali metals: They are placed in IA group, the first column on the left of the periodic table.
(b) Alkaline earth metal: They are placed in IIA group, the second column on the left of the periodic table
.(c) VIIA
(d) Aluminium: It is placed in IIIA group present on the right of periodic table.
Question 5 Name:
(a) a liquid non-metal
(b) Name two metalloids.
(c) metal which do not corrode easily
(d) Two metals which react with cold water.
(e) a non-metal which can form a positive ion,
(f) A non-metal which shows reducing property.
Answer 5
(a) a liquid non-metal Bromine
(b) Metalloids: Boron (B), Silicon (Si)
(c) Aluminium
(d) Potassium, sodium
(e) Hydrogen
(f) Carbon
Question-6
From…………………………….compound
A…..ductile.
B…conductors of electricity
C….Brittle
D….Acidic Oxide
E…Basic Oxide
F… Discharge at Anode
G….Discharge at Cathode
H Ionic Chloride
I Covalent Chloride
J. Reaction with Dil H2so4 Gives H Gas
K 1,2 or 3 valence electron
L 5,6,,7 valence electron
Answer-6
A…..ductile.
B…conductors of electricity
E…Basic Oxide
G….Discharge at Cathode
H. Ionic Chloride
J. Reaction with Dil H2so4 Gives H Gas
K. 1,2 or 3 valence electron
Page-124
Question 7
(a) Why are alkali metals kept in kerosene oil?
(b) Why is hydrogen kept in the metal activity series?
(c) Why gold ornament look new after several year of use
Answer 7
(a) Alkali metals like sodium and potassium are kept in kerosene as they react with moisture and air.
(b) Hydrogen, a non-metal, has been included in the metal activity series because it can form a positive ion. It would occupy the position based on its formation of the positive ion.
(c) Gold occupies the lowest position in the metal activity series. This means it will not react with other molecules easily. Gold is a noble element and will not form new compounds easily. Hence gold ornaments look new even after several years of use.
Question 8
From the metals copper, iron, magnesium, sodium and zinc, select a different metal in each case which
a. does not react with dilute hydrochloric acid
b. can form 2+ and 3+ ions
c. Arrange the above metals in the decreasing order of reactivity.
Answer 8
a. does not react with dilute hydrochloric acid: Copper
b. can form 2+ and 3+ ions: Iron (Fe)
c. Metals in the decreasing order of reactivity:
Na > Mg > Zn > Fe > Cu
Question 9
Which metal occurs as:
(a) A sulphide
(b) A halide
(c) A carbonate
(d) An oxide
Answer 9
(a) A metal which occurs as sulphide is lead.
(b) A metal which occurs as halide is silver.
(c) A metal which occurs as carbonate is zinc.
(d) A metal which occurs as oxide is iron.
Question 10
Distinguish between:
(a) A mineral and an ore
(b) An ore and a metallic compound
Answer 10
(a) Minerals are naturally occurring compounds of metals which are generally present with other matter such as soil, sand, limestone and rocks. Ores are those minerals from which the metals are extracted commercially at low cost and comfortably. All ores are minerals, but all minerals are not necessarily ores.
(b) Ores are those minerals from which the metals are extracted commercially at low cost and with minimum effort. A metallic compound is a compound that contains one or more metal elements. Examples: AgNO3 – Silver nitrate is a metallic compound.
Question 11
Which metal can be extracted from each one of the following ores:
(a) Bauxite
(b) Calamine
(c) Haematite
Answer 11
The metals that can be extracted from the following ores are:
(a) Bauxite- Aluminium
(b) Calamine- Zinc
(c) Haematite- Iron
Question 12
Explain the following terms:
(a) Ore
(b) Gangue
Answer 12
(a) Ore: Minerals from which, the metals may be exerted economically in reasonably pure condition.
(b) Gangue: Earthly impurities including silica, mud etc., associated with the ore are called gangue.
Metallurgy ICSE Class-10 Concise Chemistry Selina Solution Exercise-7(B)
Page-129
Question 1
Give the principles of:
(a) Hydraulic washing
(b) Forth floatation process,
(c) Electromagnetic separation
Answer 1
(a) Hydraulic washing:- The difference in the densities of the ore and the gangue is the main criterion.
(b) Forth floatation:- This process depends on the preferential wettability of the ore with oil and the gangue particles by water.
(c) Electromagnetic separation:- Magnetic properties of the ores.
Question 2
(a) Name the methods by which concentrated ore is converted to metallic oxide.
(b) State three objectives achieved during the roasting of ores.
Answer 2
(a) Roasting and calcinations
(b) (i) It removes moisture from the ore. (ii) It expels oxide. (iii) It oxidises sulphide ores to oxide ores.
Question 3
Name:
(a) The processes involved in (i) Concentration (ii) Refining of ores
(b) Two metallic oxides which cannot be reduced by carbon, carbon monoxide or hydrogen.
Answer 3
(a) The processes involved in
(i) Processes involved in concentration are:
1. Hydrolytic method
2. Magnetic Separation
3. Froth floatation
4. Leaching
(ii) Processes involved in Refining of ores are:
1. Distillation
2. Liquation
3. Oxidation
4. Electro- refining
(b) Potassium and sodium oxides cannot be reduced by carbon, carbon monoxide and hydrogen.
Question 4
Why does iron or zinc not occur free in nature?
Answer 4
Iron and zinc are quite reactive and hence they do not occur in the free state. The compounds of metals found in nature are their oxides, carbonate and sulphides.
Question 5
What do you observe when hydrogen is passed over heated copper oxide?
Answer 5
Black copper oxide is reduced to brown/red.
CuO + H2 ![]() Cu + H2O
Cu + H2O
Question 6
Compare roasting and calcination.
Answer 6
Comparison of roasting and calcinations:
| Roasting | Calcination |
| (i) The ore is heated in the excess of air.
(ii) Generally, sulphide ores are roasted, so SO2 is given off (iii) Volatile impurities are removed as oxides and the ore becomes porous and more reactive. |
(i) The ore is heated in the absence of air
(ii) Carbonate and hydrated ores are calcined and so,CO2 and water vapours are given off. (iii) Moisture and organic impurities are removed and the ore becomes porous and more reactive. |
Question 7
(a) Name an ore of Zinc.
(b) Which process is applied to concentrate it?
(c) How is concentrated ore changed to oxide?
Answer 7
(a) Ore of zinc is zinc blende (ZnS).
(b) It is concentrated by Froth floatation process.
(c) Concentrated ore is changed into oxide by heating ZnS in excess of air.
2ZnS + 3O2 ![]() 2ZnO + 2SO2
2ZnO + 2SO2
Question 8
(a)Some metallic oxides can be reduced by hydrogen, carbon and carbon monoxide and some cannot. Explain.
(b) Write balance equation for reduction of copper(II) oxide by Hydrogen (2019)
Answer 8
(a)
Oxides of highly active metals like potassium, sodium, calcium, magnesium and aluminium have great affinity towards oxygen and so cannot be reduced by carbon or carbon monoxide or hydrogen.
Metals in the middle of activity series (iron, zinc, lead, copper) are moderately reactive and are not found in oxide form. These are found in nature as sulphides or carbonate. These are first converted into oxides and can be reduced by C, CO or H2.
ZnO + C ![]() Zn + CO
Zn + CO
PbO + CO ![]() Pb + CO2
Pb + CO2
Metals low in the activity series is very less reactive and oxides of these metals are reduced to metals by heating alone.
(b) Balance equation: CuO + H2 → Cu + H2O
Question 9
How are the following metallic oxides reduced? Write equations:
(a) Iron(II) oxide
(b) Zinc oxide
Answer 9
(a) Iron(II) oxide:
4FeO + O2 ![]() 2Fe2O3
2Fe2O3
Fe2O3 + 3CO ![]() 2Fe +3CO2
2Fe +3CO2
(b) Zinc oxide is reduced by coke.
Zn O + C ![]() Zn + CO
Zn + CO
Question 10
State why aluminium is extracted from its oxide by electrolysis while copper, lead, iron by reducing agents and mercury and silver by thermal decomposition.
Answer 10
Aluminium has a great affinity towards oxygen and so cannot be reduced by carbon or carbon monoxide. So it is extracted from its oxide by electrolysis. Metals like copper, lead and iron are placed in the middle of the activity series and re moderately reactive and their oxides can be reduced by carbon, CO and hydrogen. Mercury and silver are less reactive and are placed lower in the reactivity series. The oxides of these metals are reduced to metals by heating their oxides.
Question 11
An ore on being heated in air forms sulphurous anhydride. Write the process used for the concentration of this ore.
Answer 11
The process used for the concentration of the ore is froth floatation process.
Question 12
(a) Define roasting. Name an ore on which roasting is done. Give balanced equation.
(b) Define calcination. Give example and equation for calcination.
Answer 12
(a) Roasting-is the process of heating concentrated ore to a high temperature in the presence of air. The ore zinc blende is roasted in order to get zinc oxide. Example: Zinc sulphides are oxidised to zinc oxide.
![]()
(b) Calcination is the process of heating the concentrated ore such as carbonate or hydrated oxide to a high temperature in the absence of air. Example: Metal carbonates get decomposed to produce metal oxides.
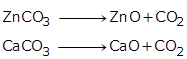
Question 13
How are the metals like sodium, potassium and calcium obtained? Give equations.
Answer 13
Sodium, potassium and calcium metals are obtained by electrolytic reduction of fused metallic salts.
Question 14
Give equation for the reduction of
(a) Copper oxide
(b) Iron (III) oxide
(c) Lead (II) oxide
(d) Zinc oxide
Answer 14
(a) Reduction of copper oxide:
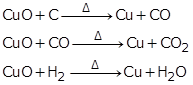
(b) Reduction of iron (III) oxide:
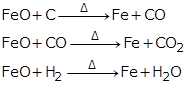
(c) Reduction of lead (II) oxide:
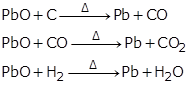
(d) Reduction of zinc oxide:
![]()
Question 15
(a) On which factors does purification of metals depend?
(b) Name the methods used for purification?
(c) With labelled diagram explain electro-refining of a particular metal
Answer 15
(a) The purification depends upon:
1. Nature of metal.
2. Nature of impurities present in the metal.
3. Purpose for which metal is to be used
(b) Methods used for purification are:
1. Distillation
2. Liquation
3. Oxidation
4. Electro-refining
(c)
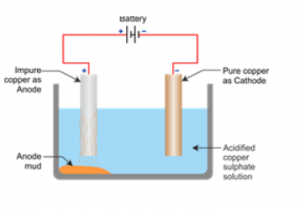
Question 16
Choose the correct option:
(a) The metal other than aluminium, which has a strong affinity for oxygen is: (A) Copper (B) Magnesium (C) Silver (D) Gold
(b) A metallic oxide which cannot reduced by normal reducing agents: (A) Zinc oxide (B) Magnesium oxide (C) Copper(II) oxide (D) Iron(III) oxide
Answer 16
(a) Option B
The metal other than aluminium which has a strong affinity for oxygen is magnesium.
(b) Option A
A metallic oxide which cannot be reduced by normal reducing agents is zinc oxide.
Question 17
Fill in the blanks:
(a) Usually …………. (sulphide/carbonate) ores are subjected to ……………. (calcination/roasting) which is done in the absence of air.
(b) Zinc blend is converted to oxide by ……… (roasting /calcination) process.
(c) Froth floatation process is generally used to concentrate ………… ores (sulphide/carbonate).
Answer 18
(a) Usually carbonate ores are subjected to calcination which is done in the absence of air.
(b) Zinc blende is converted to oxide by roasting process.
(c) Froth flotation process is generally used to concentrate sulphide ores
Exercise -7 (C), Metallurgy ICSE Class-10 Concise Chemistry Selina Solutions
Page-132
Question 1
State the position of aluminium in the periodic table.
Answer 1
Position in the Periodic Table: Period 3,Group IIIA(13)
Question 2
(a) Give the chemical names and formulae of the main ores of (i) aluminium, (ii) iron and (iii) zinc.
(b) Which impurities are present in bauxite?
(c) What is red mud, how is it removed?
Solution 2
(a)
(i) Ores of aluminium
| Name | Chemical name | Formula |
| Bauxite | Hydrated aluminium oxide | Al3O32H2O |
| Cryolite | Sodium aluminium oxide | Na3AlF6 |
(ii) Ores of iron
| Name | Chemical name | Formula |
| Red haematite | Anhydrous ferric oxide | Fe2O3 |
| Brown haematite | Hydrated ferric oxide | 2Fe2O3.3H2O |
(b) Bauxite ore contains approximately 60% aluminium oxide. The rest being sand, ferric oxide and titanium oxide.
(c) Red mud consists of ferric oxide, sand etc. left after bauxite dissolves in NaOH forming sodium aluminate and is removed by filtration.
(i)Ores of aluminium
Question 3
In order to obtain 1 tonne of aluminium, the following inputs are required: 4 tonnes of bauxite, 150 kg of sodium hydroxide and 600 kg of graphite. The aluminium compound in bauxite is aluminium oxide and the main impurity is iron (III) oxide. Aluminium is obtained by the electrolysis of aluminium oxide dissolved in cryolite.
(a) When bauxite is treated with sodium hydroxide solution, what happens to
(i) the aluminium oxide
(ii) the iron (III) oxide
(b)
(i) Name the process used for the purification of bauxite.
(ii) Write the equation for the action of heat on aluminium hydroxide
(c)
(i) Write the formula of cryolite.
(ii) Write down the word which correctly completes the following sentence. By dissolving aluminium oxide in cryolite a (conducting/non-conducting) solution is produced.
(iii) Why is so much graphite required for the electrolytic process?
(iv) Write the equation for the reaction which takes place at the cathode.
(v) What is cathode made up of?
Answer 3
(a)
(i) Aluminium oxide dissolves in sodium hydroxide and forms sodium meta aluminate leaving behind insoluble impurities consisting of ferric oxide which is removed by filtration.
(b)
(i) The process used for the purification of bauxite is Baeyer’s process.
(ii) Action of heat on aluminium hydroxide:
![]()
(c)
(i) Formula of cryolite is
![]()
(ii) By dissolving aluminium oxide in cryolite, a conducting solution is produced.
(iii). Thick graphite rods are used as the anode. The anode has to be replaced from time to time, as it gets oxidised by evolved oxygen.
(iv) Reaction at the cathode:
![]()
(v) The cathode is made of carbon.
Question 4
Aluminium is extracted from its chief ore, bauxite. The ore is first purified and then the metal is extracted from it by electrolytic reduction.
(a) Write three balanced equations for the purification of bauxite.
(b) Name a chemical used for dissolving aluminium oxide. In which state of subdivision is the chemical used?
(c) Write an equation for the reaction which takes place at the anode during the extraction of aluminium by the electrolytic process.
Answer 4
(a) Balanced equations for the purification of bauxite:
![]()
![]()
![]()
(b) Chemicals used for dissolving aluminium oxide: Fluorspar and cryolite Alumina 20%, cryolite 60%, fluorspar 20%
(c) .At anode: Al – 3e– → Al3+
Question 5
(a) A to F below relate to the source and extraction of either zinc or aluminium:
A. Bauxite
B. Coke
C. Cryolite
D. Froth floatation
E. Sodium hydroxide solution
F. Zinc blende
(i) Write down the three letters each from the above list which are relevant to
1. Zinc
2. Aluminium
(ii) Fill in the blanks using the most appropriate words from A to F.
1. The ore from which aluminium is extracted must first be treated with ………………. so that pure aluminium oxide can be obtained.
2. Pure aluminium oxide is dissolved in ….. to make a conducting solution.
(iii). Write the formula of cryolite.
Answer 5
(a)
(i)
1. Zinc
Zinc blende, Froth flotation, Coke
- Aluminium
Cryolite, Bauxite, Sodium hydroxide solution
(b)
(ii)
1. The ore from which aluminium is extracted must first be treated with sodium hydroxide solution so that pure aluminium oxide can be obtained.
2. Pure aluminium oxide is dissolved in cryolite to make a conducting solution.
(iii). Formula of cryolite is Na3AlF6.
Page-133
Question 6
Explain with reasons:
(a) In the electrolytic reduction of alumina, the graphite anode is gradually consumed.
or
Why the anode has to be replaced in this process?
(b) Roasting is carried out on sulphide ores and not on carbonates ores.
(c) Carbon can reduce lead oxide but not aluminium oxide.
(d) Electrolytic reduction is done to obtain aluminium.
(e) Why ‘food containing iron salts’ should not be cooked in aluminium utensils?
(f) A neutral gas other than oxygen is formed at the anode during electrolysis of fused alumina.
(g) why powder coke sprinkled on the top of electrolyte
Answer 6
(a) In the electrolytic reduction of alumina, the graphite (anode) is oxidized by oxygen to CO and further forms CO2, so it is consumed and has to be replaced from time to time.
2C + O2 ![]() 2CO
2CO
2CO + O2 ![]() 2CO2
2CO2
(b) Roasting provides oxygen to convert metallic sulphides into metallic oxide and SO2 which takes place when heated in excess of air.
Carbonate is converted into oxide by loss of CO2 which takes place in the absence of air and when heated strongly.
(c) Aluminium has a great affinity towards oxygen and so cannot be reduced by carbon or carbon monoxide or hydrogen whereas lead oxide can be easily reduced to metal lead by carbon.
PbO + C ![]() Pb + CO
Pb + CO
(d) Aluminium oxide is a very stable compound because of its great affinity for oxygen. It is not reduced easily by common reducing agents such as carbon or hydrogen. Hence, electrolytic reduction is done to obtain aluminium.
(e) Aluminium comes before iron in the metal activity series so it can displace iron from iron salts; thus, food containing iron salts should not be cooked in aluminium utensils.
(f) An anode is made of carbon which gets oxidised in the presence of oxygen to form carbon monoxide which is a neutral gas.
2C + O2 → 2CO
An anode is made of carbon which gets oxidised in the presence of oxygen to form carbon monoxide which is a neutral gas.
2C + O2 → 2CO
(g) Powdered coke is sprinkled over the surface of the electrolytic mixture for the following reasons:
- To reduce heat loss by radiation
- To prevent burning of the anode
Question 7
For each substance listed below, explain its significance in the extraction of aluminium:
(a) Bauxite
(b) Sodium hydroxide
(c) Cryolite
(d) Graphite (2015)
Answer 7
(a) Bauxite Aluminium is extracted from bauxite ore. It contains 60% Al2O3
(b) Sodium hydroxide The ore from which aluminium is extracted must first be treated with sodium hydroxide solution so that pure aluminium oxide can be obtained.
(c) Cryolite It lowers the fusion temperature and enhances conductivity.
(d) Graphite Thick graphite rods are used as the anode in electrolytic reduction.
Question 8
Distinguish between electrolytic methods of reduction and refining.
Answer 8
Electrolytic Reduction
(i) It is removal of oxide or halide from a metal.
(ii) Oxides of highly active metals like Na,K,Ca,Mg,Al are reduced by electrolytic reduction of their fused salts.
(iii) Oxides of these metals have great affinity for oxygen than carbon and cannot be reduced by carbon or CO or hydrogen.
Electrolytic refining of metals is the separation of residual impurities like Si and phosphorus.
(i) Presence of other metals and non-metals like Si and phosphorus.
(ii) Unreduced oxides and sulphides of metals.
It depends upon:
(i) Nature of metal
(ii) Purpose for which metal is to be obtained.
(iii) Nature of impurities present.
Impure metal is made anode while a thin sheet of pure metal is made cathode and electrolyte used is a salt of solution of a metal to be refined.
Question 9
Give three ways in which the metal zinc differs from the non-metal carbon. At least one of the differences must be a chemical difference.
Answer 9
The three ways in which metal zinc differs from the non-metal carbon is:
- Zinc has a valency2 and carbon has valency 4.
- Zinc does not form hydride but carbon does (CH4).
- Oxides of zinc areamphoteric(ZnO) whereas oxides of carbon are acidic (CO2) and neutral (CO).
Question 10
(a) Aluminium is a more active metal than iron but suffers less corrosion. Why?
(b) Explain and give reasons why aluminium vessels should not be cleaned with powders containing alkalis.
Answer 10
(a) When aluminium is exposed to the atmospheric air, it combines with oxygen and a film of aluminium oxide (Al2O3) is formed at the surface. This hard, tightly adhering film of aluminium oxide prevents corrosion.
(b) Aluminium vessels should not be cleaned with powders containing alkalis because aluminium reacts with alkalis to produce meta aluminate.
Question 11
Fill in the blanks:
(a) During the concentration of bauxite ore, aluminium goes in ………. (soluble/insoluble) part because of its …………… (acidic/basic/amphoteric) nature.
(b) In Hoope’s process, pure aluminium is collected at the …………… (top/bottom) of the electrolytic cell.
Answer 11
(a) During the concentration of bauxite ore, aluminium goes in the soluble part because of its amphoteric nature.
(b) In Hoope’s process, pure aluminium is collected at the top of the electrolytic cell.
Exercise 7 (D) ,Metallurgy Selina Concise Solution for ICSE Board Class 10th Chemistry
Page-133
Question 1
Explain the following:
(a) Zinc is used to cover iron so as to prevent rusting of iron. Why?
(b) In construction work, why is the alloy of aluminium–duralumin used rather than pure aluminium?
Answer 1
(a) Zinc is electropositive metal than iron, gets oxidized and saves iron. Also zinc forms protective layer of ZnO on iron. This layer is sticky and impervious in nature and protects the iron metal underneath from rusting.
(b) In construction work, the alloy of aluminium–duralumin is used rather than pure aluminium because of the following reasons:
The Duralumin is lighter and strong, but aluminium is light and not strong.
A Duralumin is unaffected by moist air, while aluminium gets affected by moist air.
Duralumin is corrosion-resistant, while aluminium can undergo corrosion.
Question 2
What is an alloy? How do properties of an alloy differ from its constituents?
Answer 2
Alloy is a homogeneous mixture of two or more metals or of one or more metals with certain non-metallic elements.
The properties of alloys are often greatly different from those of the components.
For example: Gold is too soft to be used without small percentage of copper.
A low percentage of molybdenum improves the toughness and wear resistance of steel.
Bell metal is more sonorous than copper or tin.
Alnico an alloy of aluminium, nickel and cobalt can lift 60 times its own mass.
These added elements improve hardness, wear resistance, toughness and other properties.
Question 3
Both Brass and bronze contain copper as major constituents. Name other elements in these alloys.
Answer 3
The other element in Brass is Zinc.
The other elements in Bronze are Tin and Zinc.
Question 4
Name an alloy of :
(a) Aluminium used in aircraft construction.
(b) Lead used in electrical wiring or electrical work in joining metals.
(c) Copper in electrical appliances or household vessels.
(d) Zinc used in simple voltaic cells.
Answer 4
(a) Duralumin
(b) Solder
(c) Brass
(d) Zinc amalgam
Question 5
What is amalgam? State its use with an example.
Answer 5
A mixture or an alloy of mercury with a number of metals or an alloy such as sodium, zinc, gold and silver as well as with some non-metals is known as amalgam.
Dental amalgam is a mixture of mercury and a silver tin alloy.
Question 6
(a) State two properties of brass that render it more useful for some purposes than its components.
(b) A metal which forms a liquid alloy at ordinary temperature.
Answer 6
(a) Two properties of brass that make it more useful than its components are:
(i) It is malleable and ductile.
(ii) It resists corrosion.
(iii) Can be easily cast.
(b) A metal which forms a liquid alloy at ordinary temperature is sodium.
Question 7
Name the constituents of:
(a) Duralumin
(b) Solder
(c) Bronze
(d) Brass
Answer 7
The constituents of
(a) Duralumin are aluminium (95%), copper (4%), magnesium (0.5%) and manganese (0.5%).
(b) Solder are lead (50%) and tin (50%).
(c) Bronze are copper (80%), tin (18%) and zinc (2%).
(d) Constituents of brass: 60–70% Cu and 40–30% Zn
Question 8
Name the following:
(a) A metal which is liquid at room temperature.
(b) A metal which is always present in the amalgam.
(c) The process of heating an ore to high temperature in the presence of air
(d )The compound formed by the reaction between calcium oxide and silica.
(e) A compound which is added to lower the fusion temperature of the electrolytic bath in the extraction of aluminium.
(f) Name an allotrope of a non-metal that allows electricity to pass through it.
Answer 8
(a) Mercury
(b) Mercury metal is always present in the amalgam.
(c) Roasting
(d )Slag
(e) Cryolite
(f) Graphite
Question 9
(a) Brass is an alloy of
(i) Copper and tin (ii) Copper and zinc (iii) Zinc and lead (iv) Lead and tin
(b) Steel is an alloy of iron and :
(i) Nickel (ii) Zinc (iii) Carbon (iv) Aluminium
(c) Reason for using aluminium in the alloy duralumin is
(i) Al is brittle (ii) Al gives strength (iii) Al bring lightness (iv) Al lower MP (2019)
Answer 9
(a)
(ii) Copper and zinc, Brass is an alloy of copper and zinc.
(b)
(iii) Carbon, Steel is an alloy of iron and carbon.
(c)
(iii) Al bring lightness
Question 10
Name the alloy used for the following purposes.
(a) Making electric circuits
(b) Making medals
(c) Making parts of watches
(d) Surgical instruments
(e) Aircraft
Answer 10
(a) Making electric circuits: Nichrome
(b) Making medals: Bronze
(c) Making parts of watches: Brass
(d) Surgical instruments: Stainless steel
(e) Aircraft: Duralumin and magnalium
Misc Exercise of Chapter 7 – Metallurgy Selina Concise Solution
Page-139
Question 1
The following is an extract from ‘Metals in the Service of man, Alexander and street/Pelican 1976’.
‘Alumina (aluminium oxide) has a very high melting point of over 2000oC so that it cannot readily be liquefied. However, conversion of alumina to aluminium and oxygen, by electrolysis, can occur when it is dissolved in some other substance’.
(a) Which solution is used to react with bauxite as first step in obtaining pure aluminium oxide?
(b) The aluminium oxide for the electrolytic extraction of aluminium is obtained by heating aluminium hydroxide. Write the equation for the reaction.
(c) Name the element which serves both as anode and cathode in the extraction of aluminium.
(d) Write the equation for the reaction that occurs at the cathode during the extraction of aluminium by electrolysis.
(e) Give the equation for the reaction which occurs at the anode when aluminium is purified by electrolysis.
Answer 1
(a) Sodium hydroxide
(b) 2Al(OH)3 ![]() Al2O3 +3H2O
Al2O3 +3H2O
(c) Graphite
(d) Reaction at cathode:
Al3+ + 3e– ![]() Al
Al
(e) Reaction at anode:
Al – 3e– ![]() Al3+
Al3+
Question 2
(a)The following is a sketch of an electrolytic cell used in the extraction of aluminium:
(i) What is the substance of which the electrodes A and B are made?
(ii) At which electrode (A or B) is the aluminium formed?
(iii) What are the two aluminium compounds in the electrolyte C?
(iv) Why is it necessary for electrode B to be continuously replaced?
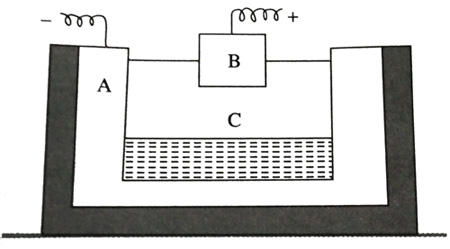
Answer 2
(a) A is made of carbon and B is thick graphite rod.
A ![]() Cathode
Cathode
B ![]() Anode
Anode
(b) Aluminium is formed at electrode A.
(c) The two aluminium compound in the electrolyte C is Na3AlF6, Al2O3.
(d) It is necessary to continuously replace electrode B from time to time as it gets oxidized by the oxygen evolved.
Question 3
State the property of the metal being utilised in the following:
| Use of metal | Property |
| Zinc in galvanisation | |
| Aluminium in thermite welding |
Answer 3
| Use of metal | Property |
| Zinc in galvanisation | Zinc oxidises more readily than iron, thus preventing the rusting of iron. |
| Aluminium in thermite welding | Aluminium is a good reducing agent. |
Question 4
Answer the following questions:
(a) Name a metal which is found abundantly in the Earth’s crust.
(b) What is the difference between calcination and roasting?
(c). Name the process used for the enrichment of sulphide ore.
(d) Write the chemical formula of one main ore of iron and aluminium.
(e) Write the constituents of the electrolyte for the extraction of aluminium.
Answer 4
(a) A metal which is found abundantly in the Earth’s crust is Aluminium
(b) Differences between calcination and roasting:
| Calcination | Roasting |
| 1. The ore is heated in the absence of air. | 1. The ore is heated in excess of air. |
| 2. Moisture and organic impurities are removed and the ore becomes porous and more reactive. | 2. Volatile impurities are removed as oxides (SO2, P2O5, As2O3) and the ore becomes porous and more reactive. |
| 3. Carbonate and hydrated ores are calcined and CO2 or water vapour is given off.
|
3. Sulphide ores are roasted, so SO2 is given off. |
(c). Froth flotation process is used for the enrichment of sulphide ore.
(d) Ores of iron
| Name | Chemical name | Formula |
| Red Haematite | Anhydrous ferric oxide | Fe2O3 |
| Brown Haematite | Hydrated ferric oxide | 2Fe2O3.3H2O |
Ores of aluminum
| Name | Chemical name | Formula |
| Bauxite | Hydrated aluminium oxide | Al3O32H2O |
| Cryolite | Sodium aluminium oxide | Na3AlF6 |
(e) Constituents of the electrolyte for the extraction of aluminium are pure alumina (, cryolite (Na3AlF6) and fluorspar ().
Previous Year Question of Chapter-7 Metallurgy Selina Concise Solution
Question 2012
(a) Name the following metals:
(i) A metal present in cryolite other than sodium.
(ii) A metal which is unaffected by dilute or concentrated acids.
(iii) A metal present in Period 3, Group 1 of the periodic table.
(b) The following questions are relevant to the extraction of aluminium:
(i) State the reason for addition of caustic alkali to bauxite ore during purification of bauxite.
(ii) Give a balanced chemical equation for the above reaction.
(iii) Along with cryolite and alumina, another substance is added to the electrolyte mixture. Name the substance and give one reason for the addition.
Answer 2012
(a)
(i) A metal present in cryolite other than sodium: Aluminium
(ii) A metal – unaffected with dilute or concentrated acids:
Aluminium
(iii) A metal present in Period 3, Group 1 of the periodic table:
Sodium (Na)
(b)
(i) Caustic alkali is added to bauxite ore during extraction as the insoluble part of bauxite is removed and the alumina component is then precipitated.
(ii) The reaction is as follows:
Al2O3.2H2O + 2NaOH →2NaAlO2 + 3H2O
(iii) Fluorspar is added along with cryolite and alumina because this helps the mixture to fuse at instead of, and the aluminium obtained at this temperature is in the liquid state.
Question 2013
(a)
| X | Y | |
| Normal electronic configuration | 2, 8, 7 | 2, 8, 2 |
| Nature of oxide | Dissolves in water and turns blue litmus red | Very low solubility in water; dissolves in hydrochloric acid |
| Tendency for oxidising and reducing reactions | Tends to oxidise elements and compounds | Tends to act as a reducing agent |
| Electrical and thermal conductivity | Very poor electrical conductor; poor thermal conductor | Good electrical conductor; good thermal conductor |
| Tendency to form alloys and amalgams | No tendency to form alloys | Forms alloys |
Using the information above, complete the following:
(i) ………….. is the metallic element.
(ii) Metal atoms tend to have a maximum of …………… electrons in the outermost energy level.
(iii) Non-metallic elements tend to form …………… oxides, while metals tend to form ……………. oxides.
(iv) Non-metallic elements tend to be …………… conductors of heat and electricity.
(v) Metals tend to …………… electrons and act as …………… agents in their reactions with elements and compounds.
Answer 2013
(a)
(i) Y is the metallic element.
(ii) Metal atoms tend to have a maximum of 3 electrons in the outermost energy level.
(iii) Non-metallic elements tend to form acidic oxides, while metals tend to form basic oxides.
(iv) Non-metallic elements tend to be poor conductors of heat and electricity.
(v) Metals tend to lose electrons and act as reducing agents in their reactions with elements and compounds.
Question 2014
(a) The main ore used for the extraction of iron is
(i) Haematite
(ii) Calamine
(iii) Bauxite
(iv) Cryolite
(b) Heating an ore in a limited supply of air or in the absence of air at a temperature just below its melting point is known as
(i) Smelting
(ii) Ore dressing
(iii) Calcination
(iv) Bessemerisation
(c) State the main components of the following alloys:
(i) Brass
(ii) Duralumin
(iii) Bronze
(d) Name the following:
(i) The property of Metals beaten into sheets.
(ii) A compound added to lower the fusion temperature of an electrolytic bath in the extraction of aluminium.
(iii) The ore of zinc containing its sulphide.
Answer 2014
(a) The main ore used for the extraction of iron is
Haematite
(b)
Calcination:- heating of ore in absent of oxygen
(c)
(i) Brass Main components of brass are copper and zinc.
(ii) Duralumin
Main components of duralumin are aluminium, magnesium, copper and manganese.
(iii). Bronze
Main components of bronze are copper, zinc and tin.
(d)
(i) Malleability – When Metal beaten into sheets.
(ii) Cryolite is added to lower the fusion temperature of an electrolytic bath in the extraction of aluminium.
(iii) . Zinc blende (sphalerite) is the ore of zinc containing its sulphide.
Question 2015
(a) Choose the most appropriate answer from the following list of oxides which fit the description. Each answer may be used only once:
[SO2, SiO2, Al2O3, MgO, CO, Na2O]
(i) A basic oxide.
(ii) An oxide which dissolves in water forming an acid.
(iii) An amphoteric oxide.
(iv) Covalent oxide of a metalloid.
(b) For each of the substance listed below, describe the role played in the extraction of aluminium.
(i) Cryolite
(ii) Sodium hydroxide
(iii) Graphite
Answer 2015
(a)
(i) Na2O
(ii) SO2
(iii) Al2O3
(iv) SiO2
(b) In the extraction of aluminium, the given compounds play the following roles:
(i) Cryolite: It lowers the fusion temperature from 2050°C to 950°C and enhances conductivity.
(ii) Sodium hydroxide:
Two roles are play with sodium hydroxide in the extraction of aluminium.
First, finely grinded bauxite (ore of aluminium) should heat under pressure with conc. caustic soda solution (NaOH solution) for 2-8 hours at 140°C to 150°C to produce sodium aluminate. The chemical equation is as follows:
Al2O3.2H2O + 2NaOH → 2NaAlO2 + 3H2O
Second, on diluting sodium aluminate with water and cooling to 50°C, sodium aluminate is hydrolysed to give aluminium hydroxide as precipitate. Here, the impurities dissolve in sodium hydroxide.
(iii) Graphite: Thick rods of graphite are suspended in the fused electrolyte. They act as an anode where oxygen gas is discharged.
Question 2016
(a) Name the solution used to react with Bauxite as a first step in obtaining pure aluminium oxide, in the Baeyer’s process. [5]
(b) Write the equation for the reaction where the aluminium oxide for the electrolytic extraction of aluminium is obtain on heating aluminium hydroxide.
(c) Name the compound added to pure alumina to lower the fusion temperature during the electrolytic reduction of alumina.
(d) Write the equation for the reaction that occurs at the cathode during the extraction of aluminium by electrolysis.
(e) Explain why it is preferable to use a number of graphite electrodes as anode instead of a single electrode, during the above electrolysis.
Answer 2016
(a) Conc. caustic soda
(b) ![]()
(c) Cryolite
(d) At the cathode: Al3+ + 3e–→ Al
(e) Anode should replace from time to time as it gets oxidise with oxygen evolve at the anode.
–: End of Metallurgy Selina Concise Chemistry ICSE Class-10 solutions :-
Return to:- Concise Selina Solutions for ICSE Chemistry Class-10
Thanks
Please Share with Your Friends

The application does not lead straightaway to the desired exercise.We have to scroll pages.
Please modify it so that we can seek answer to the desired question straightaway.
Scrolling from the beginning is tedious.
suggestion welcome