Mole Concept Dalal Simplified ICSE Chemistry Class-10 Solutions Chapter 4:- Solutions of Dr Dalal Simplified ICSE Chemistry by Dr Viraf and J Dalal for Class 10. Step by step Solutions of Dr Dalal Simplified ICSE Chemistry by Dr Viraf and J Dalal with topics -Gay Lussac ‘Law ,Avogadro numbers Mole Concept Vapour Density and Molecular Weight Percentage Composition Empirical and Molecular Formula Chemical Equations. Numericals of Mole Concept of Simplified Dalal Chemistry.
Mole Concept Dalal Simplified ICSE Chemistry Class-10
–: Select Topics :-
Part A (Mole Concept)
Mole Concept -Avogadro Law – Avogadro numbers
Vapour Density & Molecular Weight
Part B (Stoichiometry)
Empirical and Molecular Formula
Get Other Chapter Dalal Simplified ICSE Chemistry Class-10 Solutions
How to Solve Mole Concept ICSE Chemistry Class-10
Note:– Before viewing Solutions of Mole Concept And Stoichiometry by Dr Viraf and J Dalal Simplified ICSE Chemistry Solutions of Chapter-4 .Read the Chapter-4 Mole Concept And Stoichiometry Carefully to understand the concept in better way .After reading the Chapter-4 Mole Concept And Stoichiometry solve all example of your text book with ICSE Specimen Sample Paper for Class-10 Exam of Council. Focus on Numerical’s Problems. Mole Concept is the Most important Chapter in ICSE Class 10 Chemistry.Previous Year Solved Question Paper for ICSE Board
Part-A Additional Problems
Gay Lussac’ s Law — Mole Concept Dr Viraf and J Dalal Simplified ICSE Chemistry for Class-10–
Question 1.
Nitrogen reacts with hydrogen to give ammonia. Calculate the volume of the ammonia gas formed when nitrogen reacts with 6 litres of hydrogen. All volumes measured at s.t.p.
Answer:
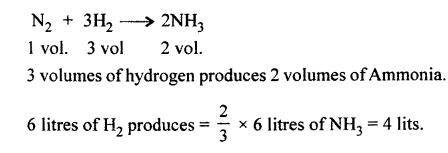
Question 2.
2500 cc of oxygen was burnt with 600 cc of ethane [C2H6]. Calculate the volume of unused oxygen and the volume of carbon dioxide formed.
Answer:
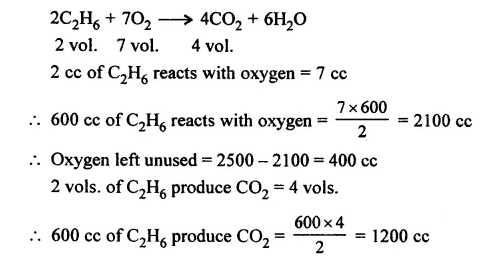
Question 3.
20 ml. each of oxygen and hydrogen and 10 ml. of carbon monoxide are exploded in an enclosure. What will be the volume and composition of the mixture of the gases when they are cooled to room temperature.
Answer:
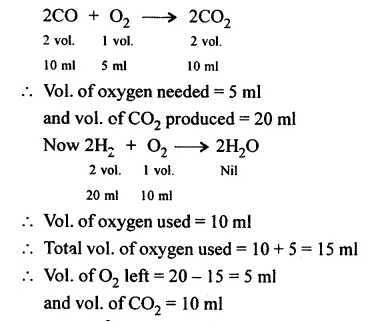
Question 4.
224 cm3 of ammonia undergoes catalytic oxidation in presence of Pt to given nitric oxide and water vapour. Calculate the volume of oxygen required for the reaction. All volumes measured at room temperature and pressure.
Answer:
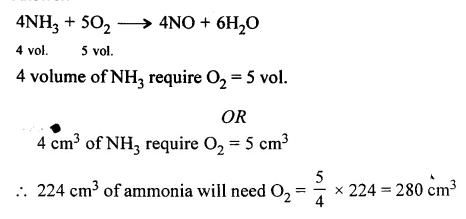
Question 5.
Acetylene [C2H2] burns in air forming carbon dioxide and water vapour. Calculate the volume of air required to completely burn 50 cm3 of acetylene. [Assume air contains 20% oxygen].
Answer:
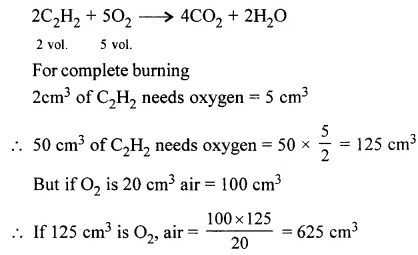
Question 6.
On igniting a mixture of acetylene [C2H2] and oxygen, 200 cm3 of CO2 is collected at s.t.p. Calculate the volume of acetylene & O2 at s.t.p. in the original mixture.
Answer:
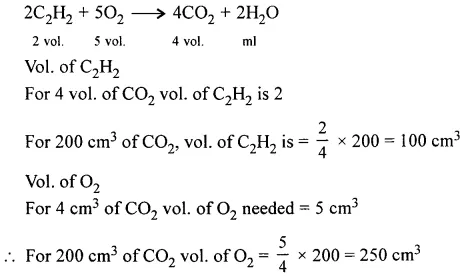
Question 7.
Ammonia is formed from the reactants nitrogen and hydrogen in presence of a catalyst under suitable conditions. Assuming all volumes are measured in litres at s.t.p. Calculate the volume of ammonia formed if only 10% conversion has taken place.
Answer:
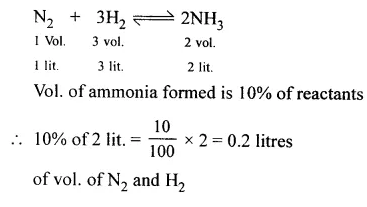
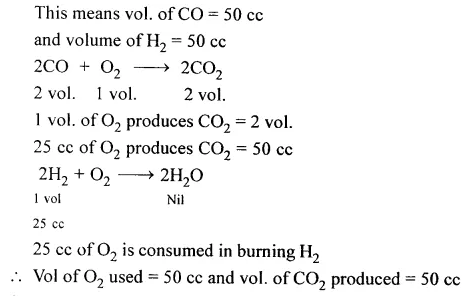
Question 8.
100 cc. each of water gas and oxygen are ignited and the resultant mixture of gases cooled to room temp. Calculate the composition of the resultant mixture. [Water gas contains CO and H2 in equal ratio]
Answer:
Mole Concept – Avogadro’S Law – Avogadro’S Number
Question 1.
The mass of 2.8 litres of C02. [C = 12, O = 16]
Answer:
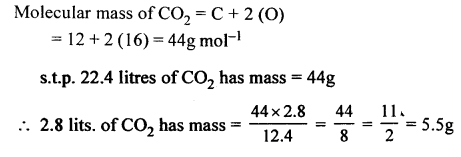
Question 2.
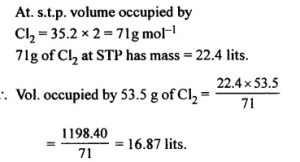
The volume occupied by 53.5g of Cl2. [Cl = 35.5]
Answer:
Question 3.
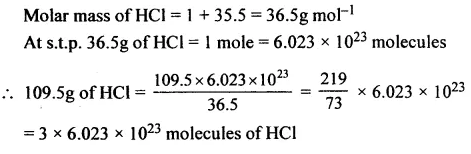
The number of molecules in 109.5 g of HCl. [H = 1, Cl = 35.5]
Answer:
Question 4.
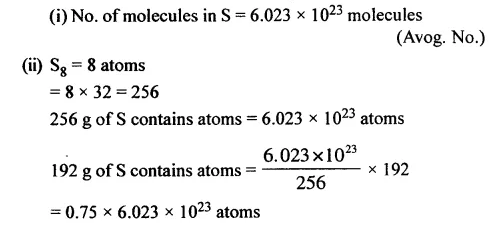
The number of
- molecules [S = 32]
- atoms in 192 g. of sulphur. [S8]
Answer:
Question 5.
The mass of (Na) sodium which will contain 6.023 × 1023 atoms. [Na = 23]
Answer:

Question 6.
The no. of atoms of potassium present in 117 g. of K. [K = 39]
Answer:

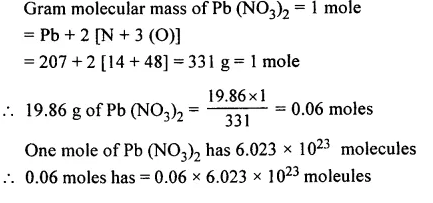
Question 7.
The number of moles and molecules in 19.S6 g. of Pb (NO3)2. [Pb = 207, N = 14, O = 16]
Answer:
Question 8.
The mass of an atom of lead [Pb = 202].
Answer:
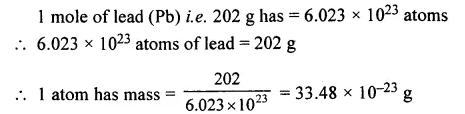
Question 9.
The number of molecules in 1 1/2 litres of water. [density of water 1.0 g./cm3. — ∴ mass of water = volume × density]
Answer:
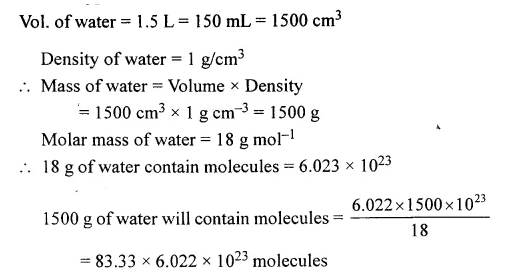
Question 10.
The gram-atoms in 88.75 g of chlorine [Cl = 35.5]
Answer:

Question 11.
The number of hydrogen atoms in 0.25 mole of H2SO4.
Answer:

Question 12.
The gram molecules in 21 g of nitrogen [N = 14]
Answer:
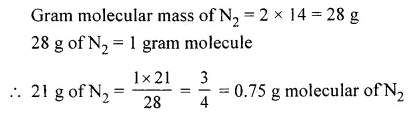
Question 13.
The number of atoms in 10 litres of ammonia [N = 14, H = 1]
Answer:
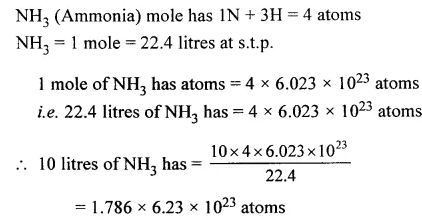
Question 14.
The number of atoms in 60 g of neon [Ne = 20]
Answer:
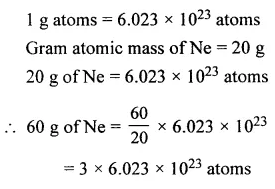
Question 15.
The number of moles of ‘X’ atoms in 93 g of ‘X’ [X is phosphorus = 31]
Answer:
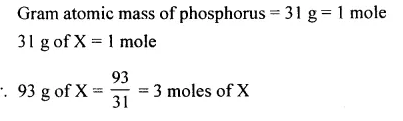
Question 16.
The Volume occupied by 3.5 g of O2 gas at 27°C and 740 mm presure. [O = 16]
Answer:
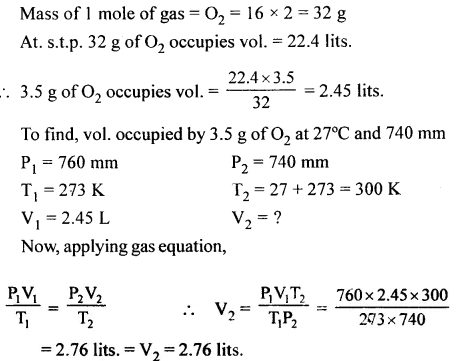
Question 17.
The moles of sodium hydroxide contained in 160 g of it. [Na = 23, O = 16, H = 1]
Answer:

Question 18.
The weight in g. of 2.5 moles of ethane [C2H6]. [C = 12, H = 1]
Answer:

Question 19.
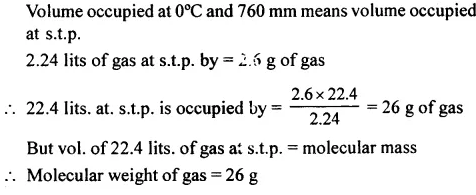
The molecular weight of 2.6 g of a gas which occupies 2.24 lits. at 0°C and 760 mm press.
Answer:
Question 20.
The gram atoms in 46 g of sodium [Na = 23]
Answer:
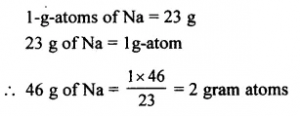
Question 21.
The number of moles of KCl03 that will be required to give 6 moles of oxygen.
Answer:
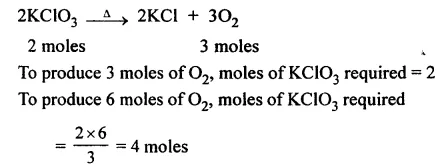
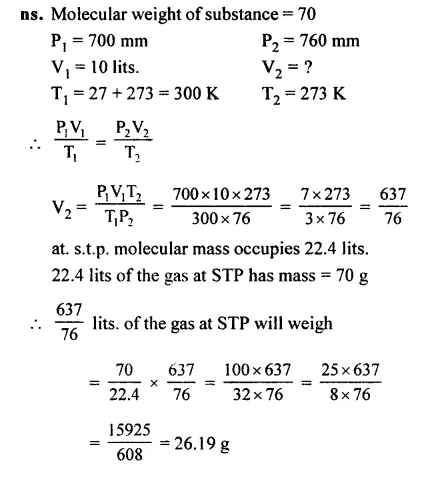
Question 22.
The weight of the substance of its molecular weight is 70 and in the gaseous form occupies 10 lits. at 27°C and 700 mm pressure.
Answer:
Question 23.
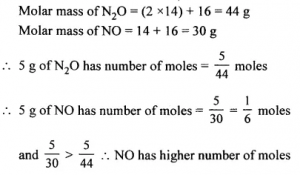
Has higher number of moles : 5 g. of N2O or 5 g. of NO [N = 14, O = 16]
Answer:
Question 24.
Has higher mass : 1 mole of CO2 or 1 mole of CO [C = 12, O = 16]
Answer:
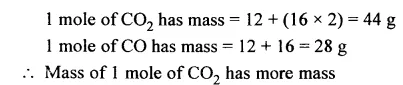
Question 25.
Has higher no. of atoms : 1 g of O2 or 1 g of Cl2 [O = 16, Cl = 35.5]
Answer:
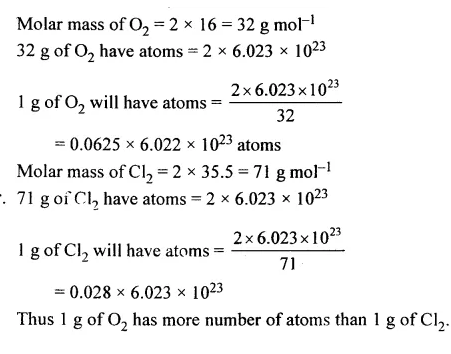
Vapour Density And Molecular Weight
Question 1.
500 ml. of gas ‘X’ at s.t.p. weighs 0.50 g. Calculate the vapour density and molecular weight of the gas. [1 lit. of H2 at s.t.p. weighs 0.09 g].
Answer:
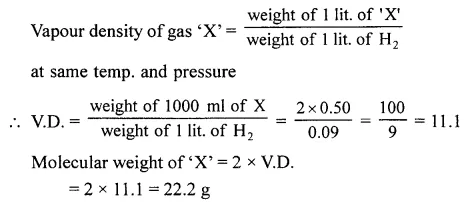
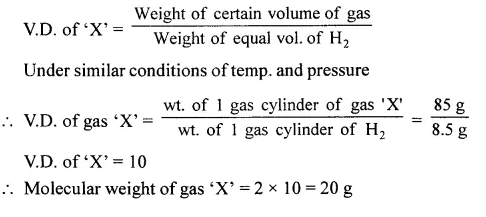
Question 2.
A gas cylinder holds 85 g of a gas ‘X’. The same cylinder when filled with hydrogen holds 8.5 g of hydrogen under the same conditions of temperature and pressure Calculate the molecular weight of ‘X’.
Answer:
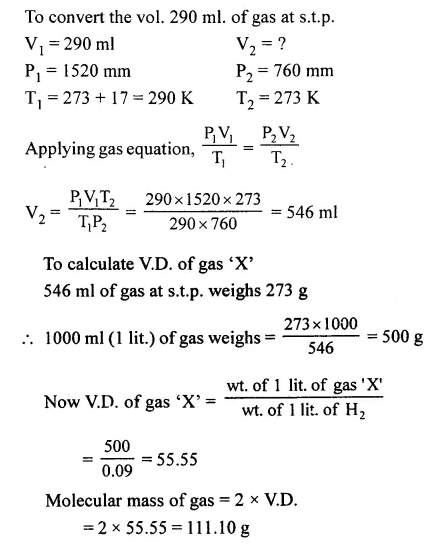
Question 3.
Calculate the relative molecular mass [molecular weight] of 290 ml. of a gas ‘A’ at 17°C and 1520 mm pressure which weighs 2.73 g at s.t.p. [1 litre of hydrogen at s.t.p. weighs 0.09 g.]
Answer:
Question 4.
State the volume occupied by 40 g of a hydrocarbon – CH4 at s.t.p. if its V.D. is 8.
Answer:
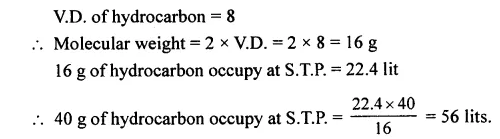
Question 5.
Calculate the atomcity of a gas X [at. no. 35.5] whose vapour density is equal to its relative atomic mass.
Answer:
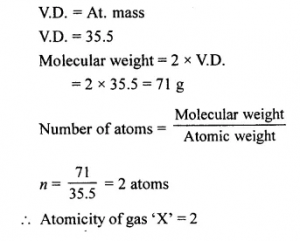
Question 6.
Calculate the relative molcular mass and vapour density of methyl alcohol [CH3OH] if 160 g. of the alcohol on vaporization has a volume of 112 litres at s.t.p.
Answer:
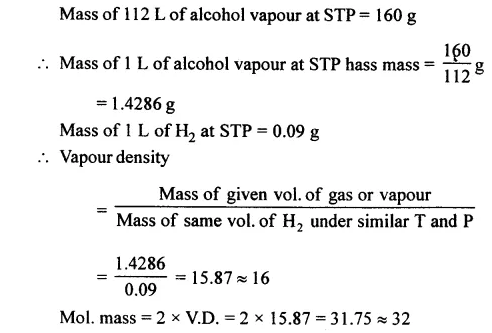
Part B (Stoichiometry)
Percentage Composition
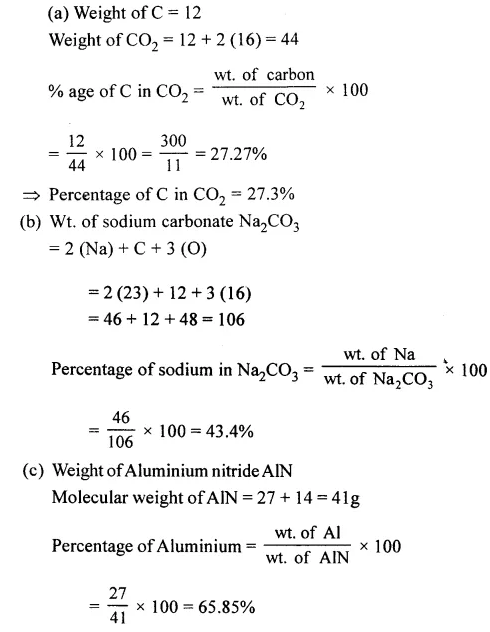
Question 1.
Calculate the percentage by weight of :
(a) C in carbon dioxide
(b) Na in sodium carbonate
(c) Al in aluminium nitride.
[C = 12, O = 16, H = 1, Na = 23, Al = 27, N = 14]
Answer:
Question 2.
Calculate the percentage of iron in K3Fe(CN)6. [K = 39,Fe = 56, C = 12, N = 14]
Answer:
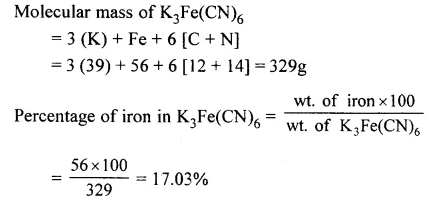
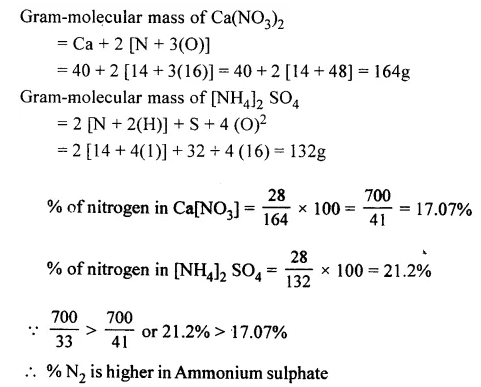
Question 3.
Calculate which of the following – calcium nitrate or ammonium sulphate has a higher % of nitrogen.[Ca = 40, S = 32, N = 14, O = 16]
Answer:
Question 4.
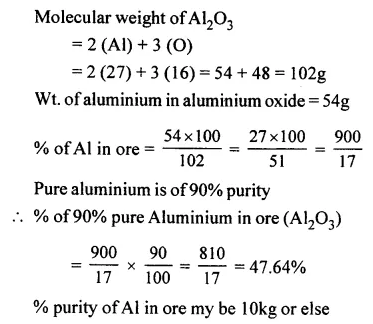
Calculate the percentage of pure aluminium in 10kg. of aluminium oxide [Al2O3] of 90% purity. [Al = 27, O = 16]
Answer:
Question 5.
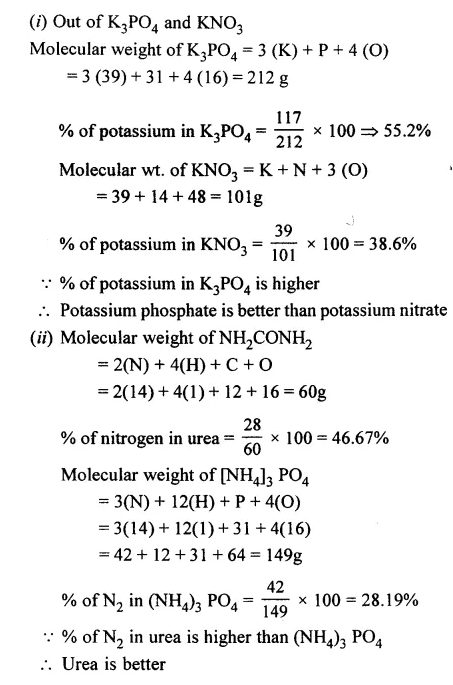
State which of the following are better fertilizers —
- Potassium phosphate [K3PO4] or potassium nitrate [KNO3]
- Urea [NH2CONH2] or ammonium phosphate [(NH4)3PO4]
[K = 39, P = 31, O = 16, N = 14, H = 1]
Answer:
Question 6.
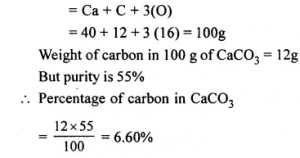
Calculate the percentage of carbon in a 55% pure sample of carbon carbonate. [Ca = 40, C = 12, O = 16]
Answer:
Question 7.
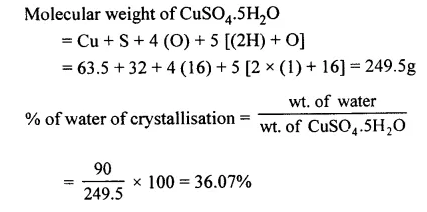
Calculate the percentage of water of crystallisation in hydrated copper sulphate [CuSO4.5H2O].
[Cu = 63.5, S = 32, O = 16, H = 1]
Answer:
Question 8.
Hydrated calcium sulphate [CaSO4xH2O] contains 21% of water of crystallisation. Calculate the number of molecules of water of crystallisation Le. ‘X’ in the hydrated compound.
[Ca = 40, S = 32, O = 16, H = 1]
Answer:
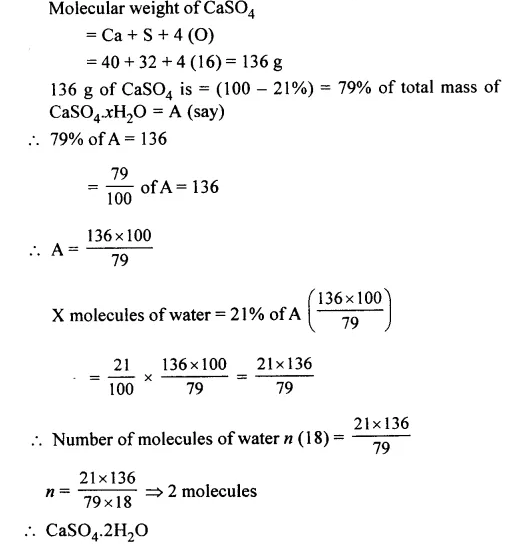
Empirical and Molecular Formula
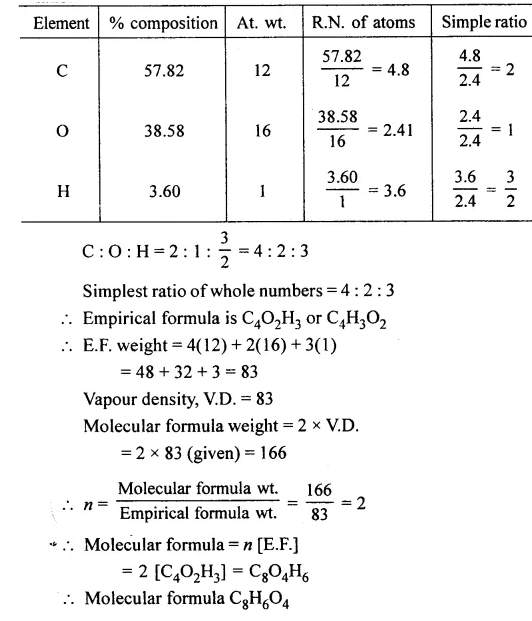
Question 1.
A compound gave the following data : C = 57.82%, O = 38.58% and the rest hydrogen. Its vapour density is 83. Find its empirical and molecular formula. [C = 12, O = 16, H = 1]
Answer:
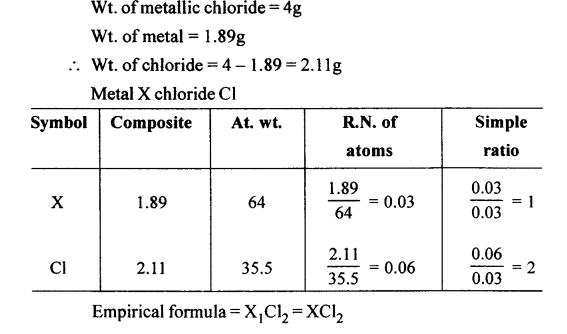
Question 2.
Four g of a metallic chloride contains 1.89 g of the metal ‘X’. Calculate the empirical formula of the metallic chloride. [At. wt. of ‘X’ = 64, Cl = 35.5]
Answer:
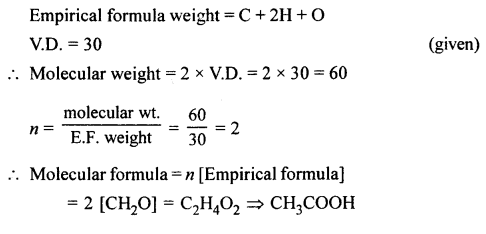
Question 3.
Calculate the molecular formula of a compound whose empirical formula is CH2O and vapour density is 30.
Answer:
Question 4.
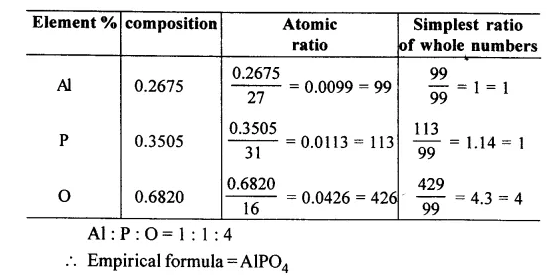
A compound has the following percentage composition. Al = 0.2675g.; P = 0.3505g.; O = 0.682g. If the molecular weight of the compound is 122 and its original weight on analysis gave the above results 1.30 g. Calculate the molecular formula of the compounds
[Al = 27, P = 31, O = 16]
Answer:
Question 5.
Two organic compounds ‘X’ and ‘Y’ containing carbon and hydrogen only have vapour densities 13 and 39 respectively. State the molecular formula of ‘X’ and ‘Y’ [C = 12, H = 1]
Answer:

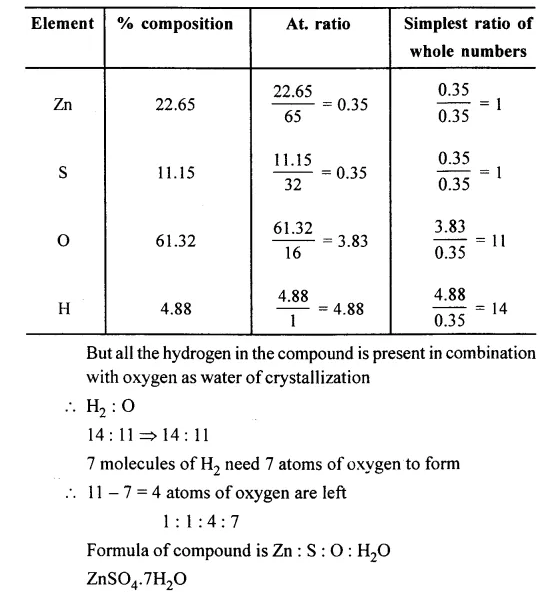
Question 6.
A compound has the following % composition. Zn = 22.65%; S = 11.15%; O = 61.32% and H = 4.88%. Its relative molecular mass is 287 g. Calculate its molecular formula assuming that all the hydrogen in the compound is present in combination with oxygen as water of crystallization.
[Zn = 65, S — 32, O = 16, H = 1]
Answer:
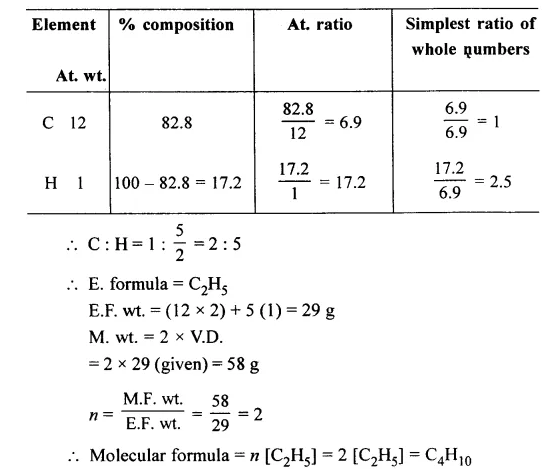
Question 7.
A hydrocarbon contains 82.8% of carbon. Find its molecular formula if its vapour density is 29 [H = 1, C = 12]
Answer:
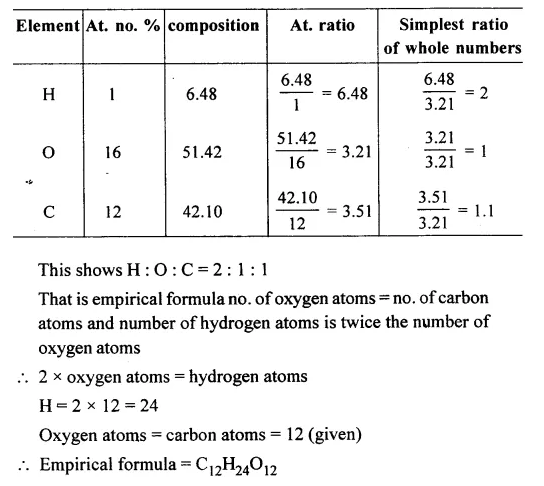
Question 8.
An organic compound on analysis gave H = 6.48% and O = 51.42%. Determine its empirical formula if the compound contains 12 atoms of carbon.
[C = 12, H = 1, O = 16]
Answer:
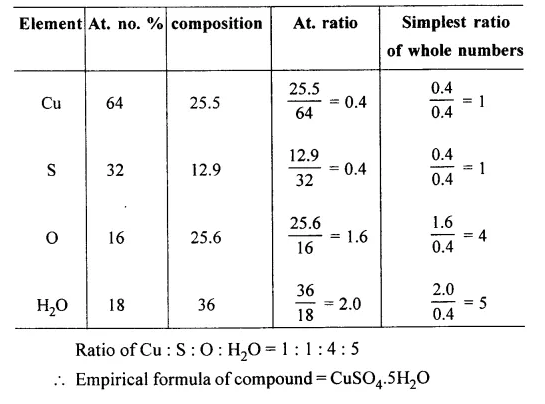
Question 9.
A hydrated salt contains Cu = 25.50%, S = 12.90%, O = 25.60% and the remaining % is water of crystallization. Calculate the empirical formula of the salt.
[Cu = 64, S = 32, O = 16, H = 1]
Answer:
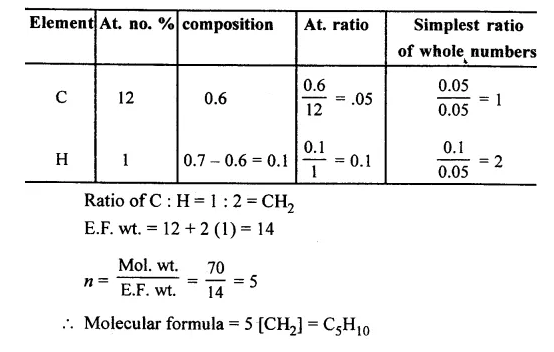
Question 10.
A gaseous hydrocarbon weights 0.70 g. and contains 0.60 g. of carbon. Find the molecular formula of the compound if its molecular weight is 70.
[C = 12, H = 1]
Answer:
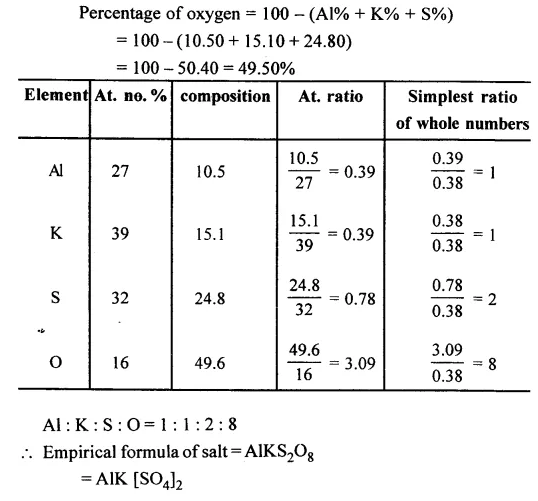
Question 11.
A salt has the following % composition Al = 10.50%, K = 15.1%, S = 24.8% and the remaining oxygen. Calculate the empirical formula of the salt.
[Al = 27, K = 39, S = 32, O = 16]
Answer:
Chemical Equations
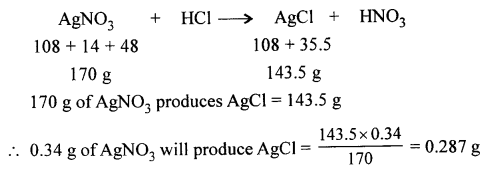
Question 1.
What mass of silver chloride will be obtained by adding an excess of hydrochloric acid to a solution of 0.34 g of silver nitrate.
[Cl = 35.5, Ag = 108, N = 14, O = 16, H = 1]
Answer:
Question 2.
What volume of oxygen at s.t.p. will be obtained by the action of heat on 20 g
KClO3 [K = 39, Cl = 35.5, O = 16]
Answer:
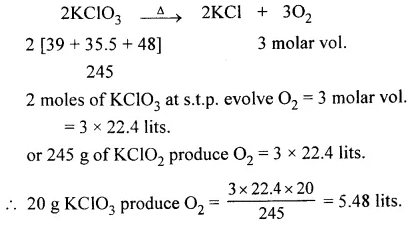
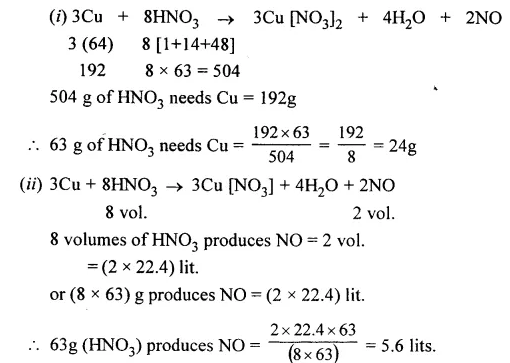
Question 3.
From the equation : 3Cu + 8HNO3 → 3Cu(NO3)2 + 4H2O + 2NO. Calculate
- the mass of copper needed to react with 63 g of nitric acid
- the volume of nitric oxide collected at the same time. [Cu = 64, H = 1, O = 16, N = 14]
Answer:
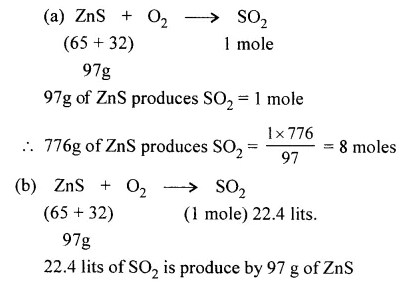
Question 4.
Zinc blende [ZnS] is roated in air. Calculate :
(a) The number of moles of sulphur dioxide liberated by 776 g of ZnS and
(b) The weight of ZnS required to produce 22.4 lits. of SO2 at s.t.p. [S = 32, O = 16, Zn = 65]
Answer:
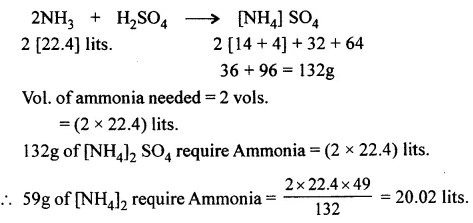
Question 5.
Ammonia reacts with sulphuric acid to give the fertilizer ammonium sulphate. Calculate the volume of ammonia [at s.t.p.] used to form 59 g of ammonium sulphate.
[N = 14, H = 1, S = 32, O = 16]
Answer:
Question 6.
Heat on lead nitrate gives yellow lead [II] oxide, nitrogen dioxide and oxygen. Calculate the total volume of NO2 and O2 produced on heating 8.5 of lead nitrate.
[Pb = 207, N = 14, O = 16]
Answer:

Question 7.
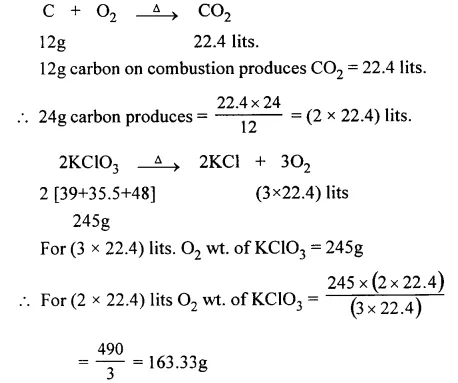
Calculate the amount of KClO3 which on thermal decomposition gives ‘X’ vol. of O2, which is the volume required for combustion of 24 g. of carbon.
![]()
[K = 39, Cl = 35.5, O = 16, C = 12].
Answer:
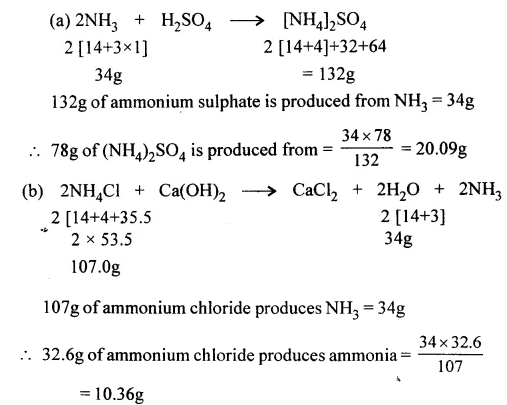
Question 8.
Calculate the weight of ammonia gas.
(a) Required for reacting with sulphuric acid to give 78g. of fertilizer ammonium sulphate.
(b) Obtained when 32.6g. of ammonium chloride reacts with calcium hydroxide during the laboratory preparation of ammonia.
[2NH4Cl + Ca(OH)2 → CaCl2 + 2H2O + 2NH3] [N = 14, H = 1, O = 16, S = 32, Cl = 35.5].
Answer:
Question 9.
Sodium carbonate reacts with dil. H2SO4 to give the respective salt, water and carbon dioxide. Calculate the mass of pure salt formed when 300 g. of Na2CO3 of 80% purity reacts with dil. H2SO4.
[Na = 23, C = 12, O = 16, H = 1, S = 32].
Answer:
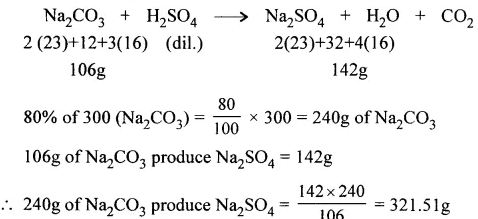
Question 10.
Sulphur burns in oxygen to give sulphur dioxide. If 16 g. of sulphur burns in ‘x’ cc. of oxygen, calculate the amount of potassium nitrate which must be heated to produce V cc. of oxygen. [S = 32, K = 39, N = 14, O = 16].
Answer:

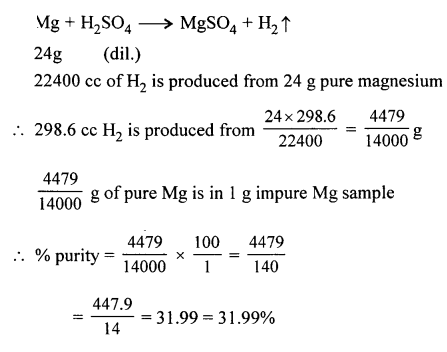
Question 11.
Sample of impure magnesium is reacted with dilute sulphuric acid to give the respective salt and hydrogen. If 1 g. of the impure sample gave 298.6 cc. of hydrogen at s.t.p. Calculate the % purity of the sample. [Mg = 24, H = 1].
Answer:
–Try Also :–

Thanks a lot it was a great help 🙏 amazing and easy to understand 👍🙏🙏🙏