Pressure in Fluids and Atmospheric Pressure Exe-4B Atmospheric Pressure and it’s Measurement Short Answer Type for Class-9 ICSE Concise Physics. There is the solutions of short Answer type Questions of your latest textbook which is applicable in 2025-26 academic session. Visit official Website CISCE for detail information about ICSE Board Class-9.
Pressure in Fluids Exe-4B Atmospheric Pressure and it’s Measurement Short Answer
(ICSE Class – 9 Physics Concise Selina Publishers)
| Board | ICSE |
| Class | 9th |
| Subject | Physics |
| Writer / Publication | Concise selina Publishers |
| Chapter-4 | Pressure in Fluids and Atmospheric Pressure |
| Exe – 4B | Atmospheric Pressure and it’s Measurement |
| Topics | Solution of Exe-4(B) Short Answer Type |
| Academic Session | 2025-2026 |
Exe-4B Atmospheric Pressure and it’s Measurement Short Answer Type
Ch-4 Pressure in Fluids and Atmospheric Pressure Physics Class-9 ICSE Concise
Page 112
Que-1: What do you understand by atmospheric pressure?
Ans: The thrust exerted per unit area of the earth surface due to column of air, is called the atmospheric pressure on the earth surface.
Que-2: We do not feel uneasy even under enormous pressure of the atmosphere above as well as around us. Give a reason.
Ans: We do not feel uneasy under enormous pressure of the atmosphere above as well as around us because of the pressure of our blood, known as blood pressure, is slightly more than the atmospheric pressure. Thus, our blood pressure balances the atmospheric pressure.
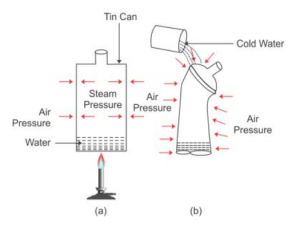
Que-3: Describe an experiment to demonstrate that air exerts pressure.
Ans: Experiment to demonstrate that air exerts pressure:
Take a thin can fitted with an airtight stopper. The stopper is removed and a small quantity of water is boiled in the can. Gradually the steam occupies the entire space of can by expelling the air from it [Fig (a)]. Then stopper is then tightly replaced and simultaneously the flame beneath the can is removed. Cold water is then poured over the can.
It is observed that the can collapses inwards as shown in fig (b).
The reason is that the pressure due to steam inside the can is same as the air pressure outside the can [Fig (a)]. However, on pouring cold water over the can, fitted with a stopper [fig (b)], the steam inside the can condenses producing water and water vapour at very low pressure. Thus, the air pressure outside the can becomes more than the vapour pressure inside the closed can.
Consequently, the excess atmospheric pressure outside the can causes it to collapse inwards.
Que-4: Explain the following :
(i) A balloon collapses when air is removed from it.
(ii) Water does not run out of a dropper unless its rubber bulb is pressed.
(iii) Two holes are made in a sealed tin can to take out oil from it.
Ans: (i) Why balloon collapses when air is removed from it
When air is removed from the balloon, the pressure inside the balloon (which was due to air in it) is much less than the atmospheric pressure outside and hence the balloon collapses.
(ii) why Water does not run out of a dropper unless its rubber bulb is pressed
Water is held inside the dropper against the atmospheric pressure because the pressure due to height column of liquid inside the dropper is less than the atmospheric pressure. By pressing the dropper we increase the pressure inside the dropper and when it becomes greater than the atmospheric pressure the liquid comes out of the dropper.
(iii) why Two holes are made in a sealed tin can to take out oil from it
There is no air inside a completely filled and sealed can. When a single hole is made to drain out the oil from the can, some of the oil will come out and due to that the volume of air above the oil will increase and hence the pressure of air will decrease. But if two holes are made on the top cover of the can, air outside the can will enter it through one hole and exert atmospheric pressure on the oil from inside along with the pressure due to oil column, and it will come out of the can from the other hole.
Pressure in Fluids and Atmospheric Pressure Exe-4B Short Class-9 ICSE
Ch-4 Pressure in Fluids and Atmospheric Pressure Physics Class-9 ICSE Concise
Page 113
Que-5: Why does the liquid rise in a syringe when its piston is pulled up?
Ans: When syringe is kept with its opening just inside a liquid and its plunger is pulled up in the barrel, the pressure of air inside the barrel below the plunger becomes much less than the atmospheric pressure acting on the liquid. As a result, the atmospheric pressure forces the liquid to rise up in the syringe.
Que-6: How is water drawn up from a well by a water pump?
Ans: In a water pump, on pulling the piston up, the pressure of air inside the siphon decreases and the atmospheric pressure on the water outside increases. As a result, the atmospheric pressure pushes the water up in pump.
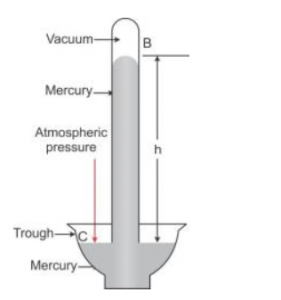
Que-7: Explain how is the height of mercury column in tube of a simple barometer is a measure of the atmospheric pressure.
Ans: In given figure, at all points such as C on the surface of mercury in trough, only the atmospheric pressure acts. When the mercury level in the tube becomes stationary, the pressure inside tube at the point A, which is at the level of point C, must be same as that at the point C. The pressure at point A is due to the weight (or thrust) of the mercury column AB above it. Thus, the vertical height of the mercury column from the mercury surface in trough to the level in tube is a measure of the atmospheric pressure.
The vertical of the mercury column in it (i.e., AB = h) is called the barometric height.
Had the pressure at points A and C be not equal, the level of mercury in the tube would not have been stationary.
(Pressure in Fluids and Atmospheric Pressure Exe-4B Short Class-9 ICSE)
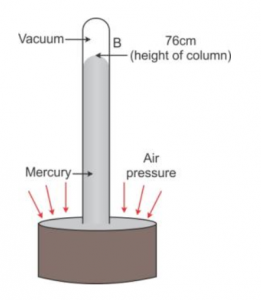
Que-8: Illustrate with the help of a labelled diagram of a simple barometer that the atmospheric pressure at a place is 76 cm of Hg.
Ans:
Que-9: Why is the barometric height used as unit to express the atmospheric pressure?
Ans: It is the atmospheric pressure acting on the surface of the mercury in the trough that supports the vertical mercury column. Hence, barometric height is used as unit to express the atmospheric pressure.
Que-10: How will you show that there is vacuum above the surface of mercury in a barometer? What name is given to this vacuum?
Ans: The space above mercury is a vacuum. This empty space is called ‘Torricellian vacuum’.
This can be shown by tilting the tube till the mercury fills the tube completely. Again when the mercury column becomes stationary, the empty space is created above the mercury column. If somehow air enters into the empty space or a drop of water gets into the tube, it will immediately vaporize and the air will exert pressure on mercury column due to which the barometric height will decrease.
Que-11: Give two reasons for use of mercury as a barometric liquid.
Ans: Two advantages of using mercury as barometric liquid:
(i) The density of mercury is greater than that of all the liquids, so only 0.76m height of mercury column is needed to balance the normal atmospheric pressure.
(ii) The mercury neither wets nor sticks to the glass tube therefore it gives the correct reading.
Que-12: Give two reasons why water is not a suitable barometric liquid.
Ans: Water is not a suitable barometric liquid because:
(i) The vapour pressure of water is high, so its vapours in the vacuum space will make the reading inaccurate.
(ii) Water sticks with the glass tube and wets it, so the reading becomes inaccurate.
Que-13: State two advantages of an aneroid barometer over a simple barometer.
Ans: Android barometer has no liquid and it is portable. It is calibrated to read directly the atmospheric pressure.
Que-14. How does the atmospheric pressure change with altitude? Draw an approximate graph to show this variation.
Ans: The atmospheric pressure decreases with an increase in the altitude.

Que-15: State two factors which affect the atmospheric pressure as we go up.
Ans: Factors that affect the atmospheric pressure are:
(i) Height of air column
(ii) Density of air
Ques-16: Why does a fountain pen leak at high altitude?
Ans: A fountain pen filled with ink contains some air at a pressure equal to atmospheric pressure on earth’s surface. When pen is taken to an altitude, atmospheric pressure is low so the excess pressure inside the rubber tube forces the ink to leak out.
Que-17: Why does nose start bleeding on high mountains?
Ans: On mountains, the atmospheric pressure is quite low. As such, nose bleeding may occur due to excess pressure of blood over the atmospheric pressure.
— : End of Pressure in Fluids and Atmospheric Pressure Exe-4B Short Answer Type Solutions :–
Return to Concise Selina Physics ICSE Class-9 Solutions
Thanks
Please share with your friends



Thanks so much sir 😊😊