Periodic Table Dalal New Simplified Class-9 ICSE Chemistry Solutions Chapter-5. We Provide Step by Step Solutions of Exercise/Lesson -5 The Periodic Table with Additional Questions , Previous Year Questions and Unit Test-5 of Dr Viraf J Dalal Middle School Chemistry Allied Publishers New Simplified Chemistry . Visit official Website CISCE for detail information about ICSE Board Class-9.
Periodic Table Dalal New Simplified Class-9 ICSE Chemistry Solutions Chapter-5
–: Select Topics :–
New Simplified Chemistry Dalal Class-9 Solutions-The Periodic Table Previous Year Questions
Question 1.(2000)
State the number of elements in Period 1, Period 2, and Period 3 of the Periodic Table.
Answer:
Period 1 is the shortest period has 2 elements Period 2 and 3 are short periods have 8, 8 elements.
Question 3.(2000)
What is the common feature of the electronic configuration of the elements at the end of Period 2 and Period 3 ?
Answer:
The elements at the end of period have 8 electrons in valence shell.
Ne = 2, 8
Ar = 2, 8, 8
Question 4.(2000)
If an element is in Group 17 [or Group 7A] is it likely to be metallic or non- metallic in character ?
Answer:
Element of group 17 or 7A has 7 electrons in valence shell hence non-metal.
Question 5.(2000)
Supply the missing word from those in brackets: If an element has one electron in its outermost energy level [shell] then it is likely to be [metallic / non- metallic].
Answer:
Supply the missing word from those in brackets: If an element has one electron in its outermost energy level [shell] then it is likely to be metallic.
Question 1.(2001)
Copy and complete the following sentences choosing the correct word or words from those given in bracket at the end of each sentence :
The similarities in the properties of a group of elements is because they have the same ____ (electronic configuration, number of outer electrons, atomic numbers.)
Answer:
The similarities in the properties of a group of elements is because they have the same electronic configuration.
Question 1.(2002)
What is meant by a Group in the Periodic Table.
Answer:
Groups : “The verticle columns from top to bottom are called groups.”
Question 2.(2002)
How many elements are there in Period 2.
Answer:
There are 8 elements in period 2.
Additional Questions of-The Periodic Table
(New Simplified Chemistry Dalal Class-9 )
Question 1.
What are elements. Give a reason why elements need to be properly classified.
Answer:
Elements : “Are pure substances made up of one kind of atoms and cannot be broken down into simpler substances.” 118 elements are there.
Reason of classification of elements :
- It makes the study of elements in an organized manner.
- To help and correlate the properties of elements with the fundamental properties of different states of matter.
- To help to define the relationship of one element with others.
Question 2.
How did the early chemists classify elements.
Why was this basis of classification discarded for future classification of elements.
Answer:
Early chemists classified the elements on the bases on their properties, nature, character, valency and whether they were metals or non-metals.
Basis of rejection :
- Some characteristics being considered, varied under different conditions.
- Certain elements resembled metallic as well as non-metallic both.
- It did not serve the purpose of classification.
Question 3.
What was Dobereiner’s basis,of classification of elements.
State Dobereiner’s Law of Triads with suitable examples.
Answer:
Basis of classification was that “there was a close relationship between the properties of an element and its atomic mass”.
Dobereiner’s Law of Triads : “When elements are arranged in order of increasing atomic masses, groups of three elements (known as triads), having similar chemical properties are obtained. The atomic mass of the middle element of the triad being equal to the arithmetic mean of the atomic masses of the other two elements.”
Examples :
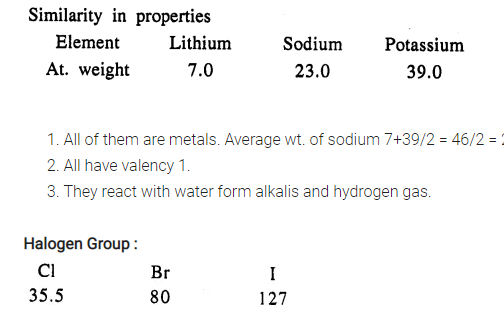
- All of them are non-metals
- All have valency one (1).
- Each reacts with water to form acid.
Question 4.
Explain why Dobereiner’s method of classilication of elements did not hold much weightage for future classification.
Answer:
Reasons for discarding the law of triads :
- Only three triads could be formed and was not holding for other elements.
- The law was not applicable even in the same family.
Question 5.
What was the basis of classification proposed by Newland.
State Newland’s Law of Octaves with a suitable example.
Answer:
Newland – Basis of classification :
If elements are arranged in ascending order of their atomic masses every eighth element had properties similar to the properties of first element, just as eighth note of a musical octave is same as its first note.
Newland’s Law of Octaves :
“When elements are arranged in order of increasing atomic masses, the properties of every eighth element (starting from any element) are repetition of the properties of that starting element.
Examples :
Li (first element)
Na (eighth element)
Ca
Properties of sodium are similar to lithium and that of Ca are similar to sodium.
Question 6.
In which way was Newland’s Law of Octaves comparable to a musical note.
Answer:
As in above Question 5.

Question 7.
How did Mendeleeff arrange the elements in the periodic table.
What was the basis of his classification.
Answer:
Mendeleeff arranged the elements in the increasing order of their atomic weights. Basis of classification was that properties of elements are periodic functions of their atomic weights.
i.e. “Elements with similar properties appear at regular intervals.”
Question 8.
State Mendeleeff’s Periodic Law.
How did Mendeleeff’s arrangement of elements correlate with periodicity of properties of elements.
Answer:
Mendeleeff’s Periodic Law :
“Physical and chemical properties of elements are periodic function of their atomic masses.”
Every ninth element resembles in properties with first element and tenth element with second and so on. That is after regular interval. This means elements in the same vertical column the properties resemble.
Question 9.
State the contributions made by Mendeleeff towards the periodic table.
Answer:
Contribution made by Mendeleeff :
- They were arranged in increasing order of atomic weights in horrizontal rows called periods in vertical columns called groups.
- Elements which are similar w.r.t. their chemical properties are grouped together and have atomic weights of nearly the same value.
- Elements in the same group had same valency and similar chemical properties.
- The properties of undiscovered elements left in the vacant gaps was predicted.
- In correct atomic weights of some of the arranged elements were corrected with the knowledge of the atomic weights of the adjacent elements.
- Number of gaps were left in the table for element not known at that time. Even predicted their properties by studying the properties of neighbouring elements. These elements were later on discovered and their properties were found to be corrects.
Question 10.
What were the defects and anomalies in Mendeleeff’s Periodic Table and how are they resolved by Moseley.
Answer:
Anomalies and Defects in Meneleeff’s Periodic Table :
- Wrong order of atomic masses of some elements could not be explained on the bases of chemical properties cobalt At. mass 58.9 (higher mass) comes first and Nickel (Ni) with lower mass (58.7) comes latter. Argon (Ar) with At. mass (39.9) precedes K (39.1).
- Position of isotopes i.e. atoms of the same element with similar chemical properties but different At. masses should have been given different places (According to Mendeleev), but were not placed separately.
- Correct position could not be assigned to Hydrogen in the periodic table. Hydrogen resembles with alkali metals of group I. Also Hydrogen resembles Halogen of group 17.
- Elements which are chemically similar such as gold and platinum have been placed in separate groups..
- It does not explain the electronic arrangement of elements.
- Elements such as copper and silver have no resemblance with alkali metals [Na, Li] but they have been placed together in first group.
Moseley arranged the elements in increasing order of atomic number and most of the defects in Mendeleev’s periodic table dissappeared.
Question 11.
How were elements arranged in the long form of the periodic table or the Modern Periodic Table. State the Modern Periodic Law and compare it with Mendeleeff’s Periodic Law.
Answer:
Long form of periodic table elements were arranged in increasing order of atomic number.
Modern Period Law :
“Properties of elements are periodic function of their atomic number”.
Comparison :
Modern Periodic Law :
- Basis is “properties of elements depend on electronic configuration.” Elements are arranged in increasing atomic number.
Mendeleeff’s Periodic Law :
- Basis is “properties of elements are periodic function of atomic mass.”
Question 12.
What are periods in a periodic table.
and What is meant by ‘period number’. What does it signify.
Answer:
Periods : “Horizontal rows from left to right in a periodic table are called periods.”
Period number : “Period numbers 1, 2, 3, 4, 5, 6, 7 signifies – no of electron shells of an element.”
i.e. period no. 2 signifies second shell from the nucleus,
Question 13.
Name the period which is the shortest period and state the number of elements present in it. State the number of elements in the second and the third periods of the periodic table.
Answer:
Shortest period is period 1.
Period 1 has 2 elements
Second period has 8 elements.
Third period has 8 elements.
Question 14.
(i) Name the elements in the correct order of their increasing atomic numbers present in the first, second and third short periods of the periodic table.
State which of the elements are – (a) metallic (b) non-metallic (c) noble gases in each of the periods 2 and 3.
(ii) State the property trends in general of elements on moving from left to right in a period of the periodic table.
Answer:
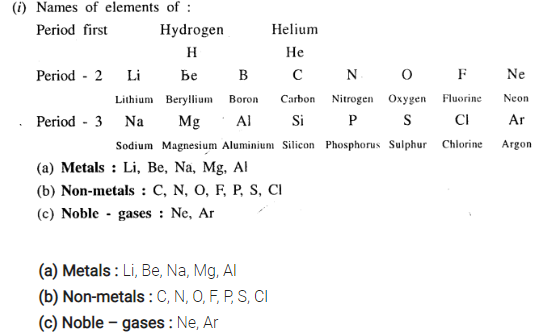
(ii) Property trends of elements from left to right in a period
(a) Number of electron shell remains the same.
(b) Valence electrons increases by one.
Transition from metallic to non-metallic character increases.
(c) Size of atom decreases.
Question 15.
What are groups in a periodic table.
State the property trends in general of elements on moving down in a group of the periodic table.
State the characteristics which remain similar on moving down in a group of the periodic table.
Answer:
Groups : “Vertical columns in a periodic table are called groups.”
Trends in a group :
- Number of shells and valence electrons : As we move down in a group the number of shells increase and valence electrons in a group remains same.
- Valency : Valency of all elements is same.
- Properties of elements : (a) Metallic character increases, (b) Non-metallic character decreases
- Atomic size increases.
Character which remains similar is physical and chemical properties, i.e. The alkali metal (Group 1-A) are very reactive Li < Na < K, Rb < Cs reactivity increases. The Halogen atoms also very reactive.
Question 16.
Write short notes on the following types of elements –
(a) alkali metals
(b) alkaline earth metals
(c) halogens
(d) noble gases
(e) transition and inner transition elements
(f) normal elements.
Answer:
(a) Alkali metals : Elements of I-A group like Li, Na, K, Rb, Cs, fr are called alkali metals because these metals react with water to form alkalis and hydrogen.
(b) Alkaline earth metals : Elements of group II-A Be, Mg, Ca, Sr, Ba, Ra are called alkali earth metals as oxides of all these metals are alkaline in nature.
(c) Halogens : Elements of groups 17 (F, Cl, Br, I, At.) are called Halogens as they react with water to form acids.
(d) Noble gases : Element of 18th or O group (He, Ne, Ar, Kr, Xe, Rn) are called noble gases as they are inert gases and have valency 6. All are mono-atomic.
(e) Transition and inner transition elements
- Transition elements : d-block elements (40 elements) which are included in groups 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12 [IB to VII-B and VIII] are called transition elements as most of them exhibit variable valency and are used as catalysts.
- Inner transition elements : f-block elements i.e. Lathanides and actinides are called inner transition elements.
(f) Normal elements OR Representative elements : “Elements of groups 1, 2, 13, 14, 15, 16, 17 which have all inner shells completely filled with electrons. Only outermost shells are incomplete are called normal elements.”
Question 17.
‘The periodic table contains elements methodically grouped together’.
State the main helpful features of the long form of the periodic table.
Answer:
The periodic table contains elements methodically grouped together like [I-A] alkali group [II-A] alkali earth group, halogens [17 OR 7-B] group, normal elements. Transition elements, noble gases [O-group] etc. If we know the properties of one member of a family, we know the properties of other members of the same family will be similar.
Moreover long form of the periodic table has 7 periods horizontal rows and 18 groups vertical columns and properties show regular gradations in periods and also in groups, i.e.
Question 18.( Periodic Table Dalal Simplified)
Name or state following with reference to the elements of the first three periods of the periodic table.
(a) The noble gas having duplet arrangement of electrons.
(b) The noble gas having an electronic configuration 2, 8, 8.
(c) A metalloid in period 2 and in period 3.
(d) The number of electron shells in elements of period 1, period 2 and period 3.
(e) The valency of elements in group 1 [IA].
(f) The group whose elements have zero valency.
(g) An alkaline earth metal in period 3.
(h) The non-metallic element present in period 3 other than sulphur and chlorine.
(i) A non-metal in period 2 having electronic configuration 2, 6.
(j) An electrovalent compound formed between an alkali metal and a halogen.
(k) A covalent compound formed between an element in period 1 and a halogen.
(l) An alkali metal in period 3 which dissolves in water giving a strong alkali.
(m) A metal in period 3 having valency 3.
(n) The bridge elements of period 3 of group 1 [IA], 2 [IIA] and 13 [IIIA].
(o) The periods which contain the inner transition elements.
(p) The formula of the hydroxide of the element having electronic configuration 2, 8, 2.
(q) The valency of the element in period 3 having atomic number 17.
(r) A non-metal in period 2 which is tetravalent.
Answer:
(a) Element is Helium
(b) Argon is a noble gas
(c) Metalloid in period 2 is B (Boron) and in period 3 is Si (Silicon)
(d) In period 1 number of electron shells is [1] In period 2 is [2], in period 3 is [3]
(e) Valency of elements in group [I-A] is +1
(f) Group is 18th or O group
(g) An alkaline earth metal in period 3 is Mg [Magnesium]
(h) is P [Phosphorus]
(i) is O [Oxygen]
(j) [NaCl] Sodium chloride
(k) HCl [Hydrogen chloride]
(l) Alkali metal is Na dissolves in water gives strong alkali NaOH
(m) Metal in period 3 is A1 [Aluminium]
(n) Elements of period 3 are Mg, A1 and Si which form bridge relationship i.e. diagonal relationship.
(o) Inner transition elements in 6th period and 7th period.
(p) Hydroxide of element [2, 8, 2] is Mg [OH], where Mg is the element
(q) Valency is 1 negative [-1]
(r) Tetravalent non-metal is carbon [C]
Unit Test Paper 5 “The Periodic Table” New Simplified Dalal ICSE Chemistry Class-9
Q.1. Select the correct answer from the words in bracket.
- He arranged elements in increasing order of atomic numbers. [Dobereiner / Moseley / Mendeleeffj
- Is a metal in period 2 having electronic configuration 2, 1. [Beryllium / Lithium / Sodium]
- Is a period having elements from atomic no. 11 to 18. [period – 1 / period – 2 / period – 3]
- The most reactive halogen from group 17. (chlorine / fluorine / bromine)
- Is the group number of the element whose atomic number is 4. [group -1 / group – 2 / group – 18]
Q.2. Fill in the blanks from the words A to F given below’.
A: Decreases
B: Increases
C: Remains same
D: Increases by one
E: Electropositive
F: Electronegative
Answer:
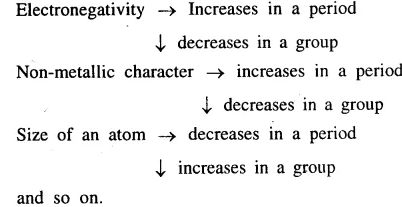
- Across a period from left to right in the Modern Periodic Table.
No. of electron shells remains same; No. of valence electrons increases by one : Electronegativity increases Character of elements changes from electropositive to electronegative. - Down a group in the Modern Periodic Table.
No. of electron shells increases by one; No. of valence electrons remains same; Electronegativity decreases Character of elements changes from electronegative to electropositive.
Q.3. Give reasons for the following.
1. Mendeleeff’s contributions to the periodic table, laid the foundation for the Modern Periodic Table.
2. Properties of elements are periodic functions of their atomic numbers and not atomic weights.
3. A transition from metallic to non-metallic elements is seen on moving from left to right in a period of the periodic table.
4. Noble gases do not form compounds readily.
5. Group 1 [IA] elements are called alkali metals.
Answer:
1. Mendeleeff found that there was close relation between atomic weights and properties of elements. He also found that when elements are arranged in increasing order of their atomic weights, the properties repeat at regular interval.
He even left some gaps for the elements not know at that time whose properties resembled the neighbouring elements. Predicted properties of eKa Aluminium (before discovery) are exactly the same as actual properties of gallium element (after discovery).
Moreover he arranged all the 63 elements known at that time. Still there were many draw-backs in this periodic table, but Mendeleeff laid the foundation of modern periodic table.
2. Atomic number :
Number of electrons in an atom, it helps in arranging the elements according to their electronic configuration so the bases of atomic number is better than atomic mass.
3. In a period valence electrons increase from 1 to 7. But shell remains the same. Element having valence electron 1, 2, 3 lose electrons and are metals on the left. And elements having 5, 6, 7 valence electrons gain electrons and become non – metals are on the right of periodic table.
Hence transition from metallic to non-metallic character is seen from left to right a period.
4. Noble gases do not form compounds readily as noble gases have their outer most orbit completely filled and have stable configuration.
5. Elements of I-A group are metals, react with water to form alkalis.
Q.4. State the following.(Periodic Table Dalal Simplified )
- The grotip to which the -element with electronic configuration of 2, 8, 2 belongs.
- The group from the groups 1 [IA], 2 [IIA], 16 [VIA] and 17 [VIIA] whose elements are most electronegative.
- The group which contains highly electropositive’ metals including sodium.
- group whose elements are unreactive or inert.
- The group which contains highly reactive electronegative non-metals including chlorine.
Answer:
-
- Element [2, 8, 2] belongs to group 2 or [II-A]
- Most electronegative 17 [VII-A].
- 1 [I-A] group contain highly electropositive metals.
- Inert elements group 18 [0 group].
- Highly reactive group 17 [VII-A],
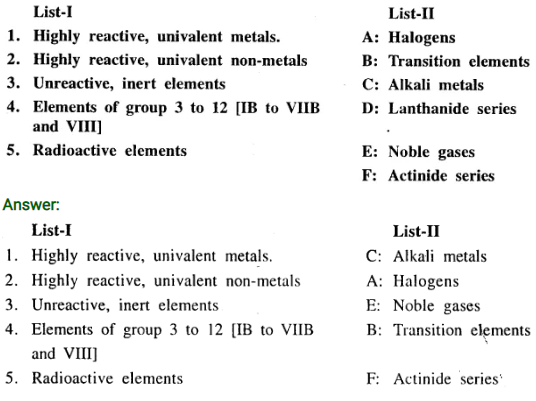
Q.5. Match the elements of List-I with their type from List-II.
Q.6. Complete the table pertaining to the following elements given in Column 1.
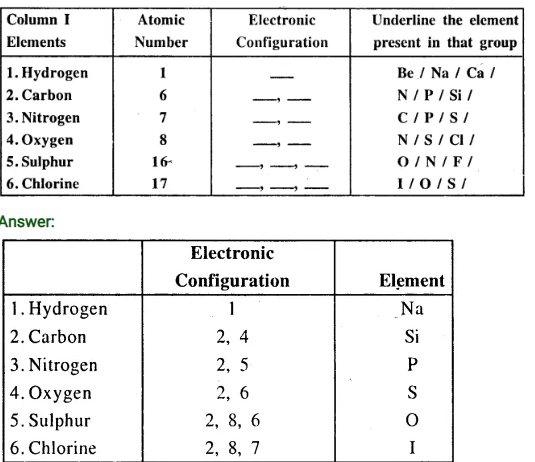
.– : End of The Periodic Table Solutions :–
Return to New Simplified Dalal ICSE Chemistry Class-9 Solutions
Thanks
Share with your friends




Thanks a lot these helped me alot
ok please keep in touch
Thanks a lot
Really a good site
thanks
Thanks a lot
Really good site
thanks for response