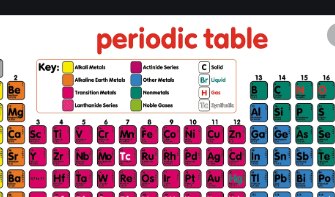
Periodic Table Dalal Simplified ICSE Chemistry Class-10 Solutions Chapter 1. Solutions of Dr Dalal Simplified ICSE Chemistry Chapter-1 Periodic Table by Dr Viraf and J Dalal for Class 10. Step by step Solutions of Dr Dalal Simplified ICSE Chemistry by Dr Viraf and J Dalal of Periodic Table Dalal Simplified Chemistry.
Periodic Table Dalal Simplified ICSE Chemistry Class-10
How to Solve Periodic Table ICSE Chemistry Class-10
Note:– Before viewing Solutions of Periodic Table by Dr Viraf and J Dalal Simplified ICSE Chemistry Solutions of Chapter-1 .Read the Chapter-1 Periodic Table Carefully to understand the concept in better way .After reading the Chapter-1 Periodic Table solve all example of your text book For more practice on Periodic Table try to solve exercise of other publications also such as Goyal Brothers Prakshan Chemistry / Concise Selina Publications Chemistry.
Additional Questions Periodic Table Dalal Simplified ICSE
Question 1.
State the fundamental property on which the modern periodic table or long form of periodic table is based.
Answer:
Properties of the elements are periodic function of their atomic number.
Question 2.
State the important salient features of the modern periodic table. State how separation of elements and periodicity of elements forms an important feature of the modern periodic table.
Answer:
Physical and chemical properties of elements are periodic functions of their atomic number.
Salient Features of Modern Periodic Table –
- This table has a eighteen veritcal columns called groups or families.
- The groups are IA to VILA, IB to VIIB, VIII (three columns) and zero group.
According to the latest recommendations of the International Union of Pure and Applied Chemistry (I.U.P.A.C.), the groups are numbered 1-18. - The group number is assigned to an element depending upon the number of the valence electrons.
- Elements included in the same group of the periodic table have similar outer electronic configuration and constitute a family of chemically similar elements.
Question 3.
What are ‘periods’. State the correlation of a period number with the elements of that period.
Answer:
Modem periodic table has seven horizontal rows called periods. There is a gradual change in properties with increase in atomic number in the periodic table.
Question 4.
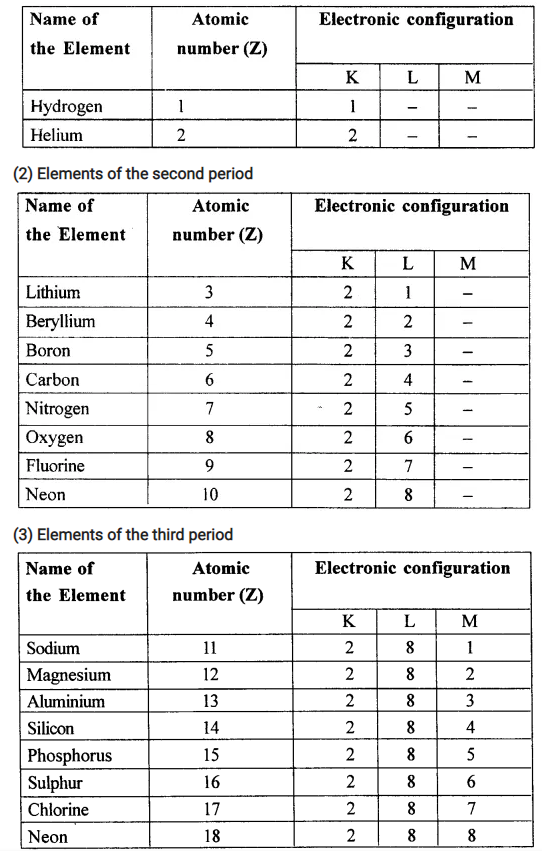
Name the elements in correct order of their increasing atomic number present in the first, second and third short periods of the periodic table. State each elements electronic configuration.
Answer:
(1) Elements of first period
Question 5.
Give a reason why
(a) completion of each period is logical
(b) period-2 elements are ‘called bridge elements’.
Answer:
(a)
Completion of each period is logical since each period begins with Group 1 element having one electron in outermost shell and ends with Group 18 element having filled outermost shell with 8 electrons.
(b)
Li, Be, B, C are called Bridge elements because they show similarties in properties diagonally with the period of the next group.
Question 6.
State the property trends in general on moving from left to right in a period of the periodic table.
Answer:
The property trends in general on moving from left to right in a period of the periodic table are:
- Number of valence electrons increases by one
- Metallic character decreases while non-metallic character increases (with the exception of Noble gases).
- Atomic radius – decreases.
- Electron affinity – increases.
- Electronegativity – increases (with the exception of Noble gases).
- Ionisation potential – increases.
Question 7.
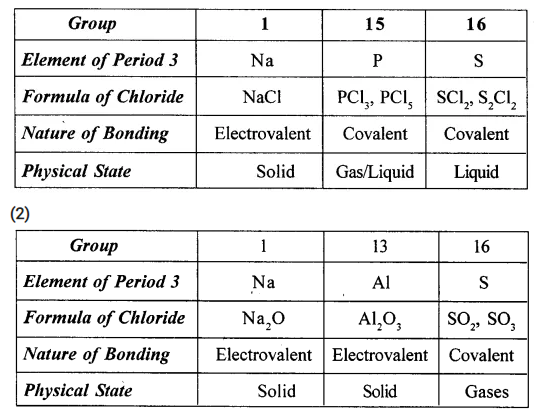
State
[1] the bonding and state of chlorides of period-3 – group 1 [IA], 15[VA], 16 [VIA] and
[2] the bonding and character of oxides of period-3 – group 1 [IA], 13[IIIA] and 16[VLA].
Answer:
(1)
Question 8.
What are ‘groups’ of the Modern Periodic Table ? What does the ‘group number’ signify.
Answer:
There are 18 vertical columns in the Modem periodic table. These vertical columns are called Groups.It signifies the same electronic configuration and similar properties. .
Question 9.
State the type of elements present in
(a) group 1[IA]
(b) group 2 [IIA]
(c) group 3 to 12 [IB to VIIB and VIII]
(d) group 13 to 16 [HIA to VIA]
(e) group 17 [VIIA]
(f) group 18 [0].
Answer:
(a) Group-1 [IA] Alkali metals
(b) Group-2 [IIA] Alkaline Earth metals
(c) Group-3 to 12 IB to VII B to VIII – Transition elements- metals.
(d) Group 13-16 [III B to VI A] – Post Transition elements.
(e) Group 17 (VII A) Halogens.
(f) Group 18 (0) Noble gases.
Question 10.
What are transition elements and inner transition elements. State the position of the inner transition elements. State why noble gases are considered unreactive elements.
Answer:
Transition elements – Elements belonging to Group 3 to 12 are called transition elements. Transition elements consists of metals lying between strongly elctropositive metals on a left and least electropositive elements on the right. They all have similar properties.
Inner transition elements: Inner transition elements are the elements belonging to Group 3 in 6th and 7th period.
They form two series i.e. Lanthanide series and Actinide series.
They consists of two Horizontal rows of metals at the bottom of the table.
The gases of zero group (Noble gases) are unreactive because they have a stable electronic configuration.
Question 11.
State the characteristics which remain similar and those which show a transition on moving down a sub-group.
Answer:
Characteristics which remain similar on moving down a group/ sub-group are:
- Valency of electrons.
- Chemical properties.
Characteristics which show a transition or moving down a group are:
- Metallic character increases down the group.
- Number of electron shells increases down the group.
- Atomic size increases down the group.
- Ionisation potential decreases down the group.
- Electron affinity decreases down the group.
Question 12.
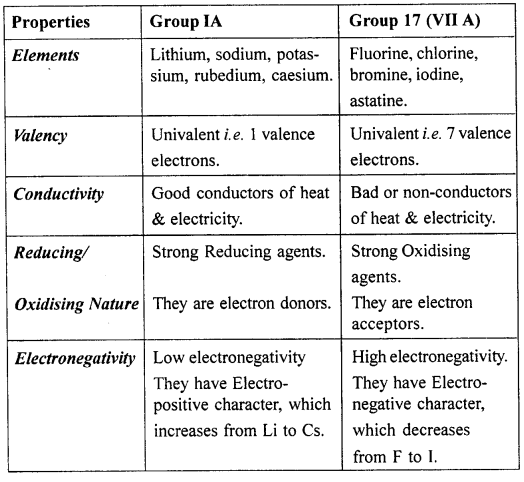
Compare the properties of the elements of group 1[IA] i.e. alkali metals and group 17 , halogens.
Answer:
Question 13.
Explain the term (a) periodicity in properties of elements (b) periodic properties (c) periodicity of elements.
Answer:
(a) Periodicity in properties of elements means occurrence of characteristic properties at definite intervals in the modem periodic table, when elements are arranged in increasing order of their atomic number.
(b) Periodic properties: The properties which appear at regular intervals in the periodic table are called periodic properties.
Periodic Properties are:
- Atomic radii Ionisation potential
- Electron affinity Electro negativity
- Non Metallic and Metallic character.
- Density Melting and boiling points.
(c) Periodicity of elements: Occurrence of elements with similar chemical properties at definite intervals when elements are arranged in increasing order of their atomic numbers is called periodicity of elements.
Question 14.
State the reasons for periodicity of elements in periods and groups.
Answer:
Reasons for Periodicity in properties in periods and groups.
- After definite intervals of atomic number, similar valence shell electronic configuration occurs.
- Properties of elements depend upon the number and arrangement of electrons in various shells including valence shells.
- In the same period, sub-group, increases or decreases, in a particular property is due to gradual change in electronic configuration in the arranged elements.
Question 15.
Explain the meaning of the following periodic properties:-
[a] Atomic radius
[b] Ionisation potential
[c] Electron affinity
[d] Electronegativity
[e] Non-metallic and metallic character.
Answer:
(a) Atomic radii: It is distance between the center of the nucleus and outer-most shell of the atom.
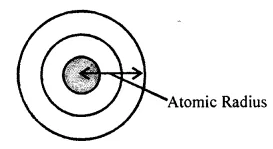
(b) Ionisation potential (LP): It is the amount of energy required to remove an electron from the outer most shells of an isolated gaseous atom.
(c) Electron affinity: It is an amount of energy released when an atom in the gaseous state-accepts an electron to form an anion.
(d) Electro-negativity: It is the tendency of an atom to attract electrons to itself when combined in a compound.
(e) Non-metallic and Metallic character: In terms of electron loss or gain, an element is a Non-metal if it gains one or more electrons if it gains one or more electrons, and a Metal if it loses one or more electrons.
Question 16.
State the factors which affect the atomic size of elements in a periodic table. In period 2 from left to right, state which element has the largest atomic size and which element has the smallest, giving reasons.
Answer:
Factors affecting the atomic size are:
- Number of shells: As number of shells increases, atomic size i.e. the distance of the outermost shell from the nucleus also increases.
- Nuclear charge: As nuclear charge increases, atomic size decreases. This is because a greater nuclear charge means a greater attraction between the nucleus and the electrons in the outermost shell.
Lithium(Li) has the largest Atomic size and Fluorine (F) has the smallest Atomic size in period
This is because on moving across a period, number of shells remains the same but the nuclear charge increases by one at each step.
Question 17.
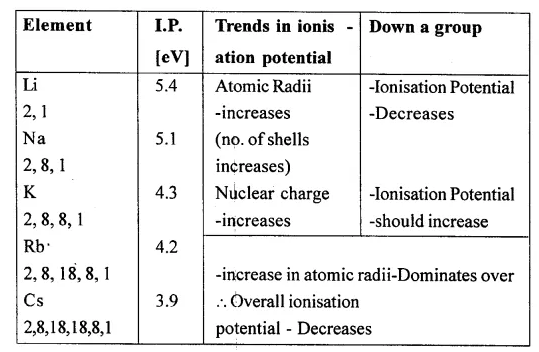
Explain the trend in atomic radii on moving down a group, with reference to the alkali metals in Group 1 [IA].
Answer:
Atomic radii increases
Question 18.
State the factors which influence or affect the ionisation potential of elements in a periodic table.
Answer:
The factors which influence the ionisation potential of an element are:
- As Atomic size increases ionisation potential decreases.
- As Nuclear charge increases ionisation potential increases.
Question 19.
Explain the trend in general of ionisation potential of elements
(1) on moving from left to right across a period
(2) on moving down a group.
Give reasons for the change in the periodic trend in each case.
Answer:
(a) Ionisation potential increases across a period left to right.
Reason:
The nuclear charge increases –
The nuclear attraction on the outer electrons increases.
Hence the outer electrons are more firmly held.
∴ Ionisation potential increases.
(b) Ionisation potential decreased down group.
Reason: Atomic size increases
The nuclear attraction on the outer electrons decreases.
Hence the outer electrons are loosely held.
∴Ionisation potential decreases.
Question 20.
State the factors which affect (a) electron affinity (b) electronegativity of elements in a periodic table.
Answer:
(a) Electron affinity is the tendency of an atom to accept electrons.
The Factors affecting the electron affinity are:
- Atomic size: As the atomic size increases electron affinity decreases.
Reason: A small atom takes up electrons more easily than a large atom since nucleus has greater attraction on the nucleus.
- Nuclear charge: As the nuclear charge increases electron affinity increases.
Reason: More the nuclear charge, more is the tendency of an atom to accept electrons.
(b) Electronegativity is the tendency of an atom to pull the shared pair of electrons towards itself in a covalent compound.
The factors affecting electronegativity an element are:
- Atomic size: More the atomic size less is the electronegativity.
Reason: A small atom will pull the shared pair of electrons more than a large atom. - Nuclear charge: More the nuclear charge, more is the electronegativity.
Reason: A greater nuclear charge means more attraction on the shared pair of electrons.
Question 21.
Explain the trend in general of
(1) electronaffinity
(2) electronegativity of elements
- on moving from left to right across a period;
- on moving down a group. Give reasons for the change in each periodic trend.
Answer:
(1) General variations of electron affinity in the Periodic table:
Electron affinity, in general, increases with increase in nuclear charge and decreases with increase in atomic size.
- Variations in a Period: On moving across a period from left to right, atomic size decreases while nuclear charge increases. Both of these factors increase the electron affinity. Thus electron affinity, in general, increases from left to right on moving across a period in the period table.
- Variation in a Group: On moving down a group from top to bottom, both atomic size and nuclear charge increase. However, the effect of increase in atomic size dominates over the effects of increase in nuclear charge. Thus, electron affinity, in general, decreases from top to bottom on moving down a group in the periodic table.
(2) General variations of electronegativity in the periodic table:
Electronegativity increases with increase in nuclear charge and decreases with increase in atomic size.
- Variations in a Period : On moving across a period from left to right atomic size decreases while nuclear charge increases. Both of these factors increase the electronegativity. Thus electronegativity, in general, increases from left to right on moving across a period in the periodic table.
- Variations in a Group: On moving down a group from top to bottom both atomic size and nuclear charge increase. However, the effect of increase in atomic size dominates over the effect of increase in nuclear charge. Thus electronegativity, in general, decreases from top to bottom on moving down a group in the periodic table.
Question 22.
With reference to the alkali metals in Group 1 [IA] and the halogens in 17 [VIIA] explain the trend in ionisation potential, electron affinity and electronegativity on moving down the groups in the periodic table.
Answer:
(1) Trends in periodic properties (Group 1)
Group 1 elements are Li (At. no. = 3), Na (At. no. = 11), K(At. no. = 19), Rb(At. no. = 37),
Cs (At. no. = 55).
(a) Trend in ionisation potential:
On moving down the group, ionisation potential decreases, i. e., ionisation potential decreases in the order: Li > Na > K > Rb > Cs.
Reason: On moving down the group, atomic size, as well as, nuclear charge increases.
However, the effect of increase in atomic size dominates over the effect of increase in nuclear charge. As such ionisation potential decreases down the group.
(b) Trend in electron affinity:
On moving down the group, electron affinity decreases, e., electron affinity decreases in the order:
Li > Na > K > Rb > Cs.
Reason: Same as that in ionisation potential.
(c) Trend in electronegativity:
On moving down the group, electronegativity decreases, e., electronegativity decreases in the order : Li > Na > K > Rb > Cs.
Reason: Same as that in ionisation potential.
(2) Trends in periodic properties (Group 17):
Group 17 elements are F (At. no. = 9), Cl (At. no. = 17), Br (At. no. = 35) and I (At. no. = 53).
(a) Trend in Ionisation potential:
On moving down the group ionisation potential decreases, e. ionisation potential decreases in the order : F > Cl > Br > I.
Reason: On moving down the group, atomic size number of shells as well as nuclear charge increase. However, the effect of increase in atomic size dominates over the effect of increase in nuclear charge. As such ionisation potential decreases down the group.
(b) Trend in electron affinity:
On moving down the group, electron affinity, in general, decreases. However, the actual order of change in electron affinity is : F < Cl > Br > I. Reason : Same as in case of ionisation potential.
(c) Trend in electronegativity:
On moving down the group, electronegativity decreases, e., electronegativity decreases in the order: F > Cl > Br > I.
Reason: Same as in case of ionisation potential
Question 23.
State the factors which affect the metallic and the non- metallic character of elements in a periodic table.
Answer:
Factors affecting metallic/non-metallic character in a periodic table:
(1) When atomic size increases, metallic character increases
(2) When ionisation potential increases, non-metallic character increases.
Question 24.
Explain the trends from metallic to non-metallic character of the different elements the first three periods.
Answer:
The ease with which an element loses one or more of its electrons is called its metallic or electropositive character. On the other hand, the ease with which an element gains one or more electrons is called its non-metallic or electronegative character. It mainly depends upon atomic size and ionisation potential.
Trends in metallic/non-metallic character in the first three periods.
- Atomic radii – decreases
Metallic character – Decreases
Non metallic character – Increases
- Ionisation potential – Increases
Metallic character – decreases
Non-metallic character – increases
Question 25.
Explain with reasons the trends in metallic and non- metallic character down a group.
Answer:
The ease with which an element loses one or more of its electrons is called its metallic character. On the other hand, the ease with which an element gains one or more electrons is called its non-metallic character. It mainly depends upon atomic size and ionisation potential. More the atomic size and lesser the ionisation potential, more is the metallic character. However, the reverse is true for non-metallic character of an element.
In a group atomic size increases while ionisation potential decreases as we move down a group. Thus, down a group metallic character increases while non-metallic character decreases.
Question 26.
State how density and melting points of elements varies across a period and down a group.
Answer:
Density, in general, increases with increase in atomic number. As such density increases as we move down a group from top to bottom.
Trend in melting point is different for metals and non-metals as is clear from the change in melting point in Group I or Group 2 (groups which contain only metals), and Group 17 or Group 18 (groups which contain only non-metals). In groups which contain . only metals, m.p. decreases down the group while in groups which contain only non-metals, m.p. increases down the group. Thus in group I, m.p. is in the order ; Li > Na > K > Rb > Cs while in Group 17, m.p. is in the order: F2>Cl2<Br2<I2
Question 27.
State the general trend in periodicity in properties of oxides, hydroxides, oxy-acids and hydrides of compounds of elements across a period and down a group.
Answer:
Oxides, hydroxides and oxy-acids:
Metals form basic oxides. If these oxides are soluble in water, they form metal hydroxides (alkalies). Metalloids form amphoteric oxides, which show both acidic and basic character : Non-metals generally form acidic oxides. These oxides, when dissolved in water, form oxy-acids.
Variations in a Period:
On moving from left to right in a Period, metallic character decreases while non-metallic character increases. Thus elements of Group 1, 2 and 13 form basic oxides and their basic character decreases along the period. Elements of Group 14, 15, 16 and 17 generally form acidic oxides. The acidic character of these oxides increases along the Period. For example, acidic/basic character of oxides
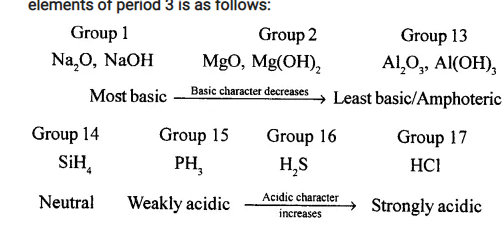
Elements of group 18 do not form oxides.
Variation in a Group:
On moving down a Group, metallic character increases while non-metallic character decreases. Thus, on moving down the group basic nature of oxides and hydroxides increases (for Group 1,2 and 13), while acidic nature of oxides and oxy-acids generally decreases (for Group 14,15,16 and 17).
For example, in Group 2, Ba(OH)2 is a much stronger base than Mg(OH)2, while in Group 15, H, PO4 is a much weaker acid than HNO3.
Hydrides:
Metals of Group 1, 2 and 13 form ionic hydrides which are strong reducing agents. Non-metals of Group 14 to 17 form covalent hydrides which can be acidic, basic or neutral.
Along a period, the acidic character of these hydrides increases.
For example, in 3rd period.
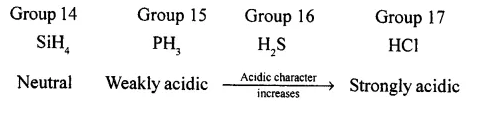
Down the group, the basic nature of these hydrides decreases while acidic nature increases.
For example in Group 15,

Question 28.
State the relation between atomic number and atomic mass for light State which elements are considered radioactive giving reasons.
Answer:
For light elements,
Atomic mass = 2 x Atomic number
In other words, for light elements
Number of neutrons (n) = Number of protons (p)
Thus for light elements, nip 1
For example, in 10Ne20, /? = 10 ; « = 20 -10=10. Thus nip = 10/10=1
For heavier elements, nip > 1. For example, in 17CI37, p ~ 17 and n = 37- 17 = 20
Thus, nip = 20/17 = 1.176
If nip > 1.5, the nucleus of the elements becomes unstable and the element becomes radioactive. For example, in radioactive 92U235, p = 92 and n = 235 – 92 = 143.
Thus, ntp= 143/92= 1.554.
Question 29.
Answer the following :-
(a)
Name or state the following with reference to the elements of the modern periodic table.
1. The alkali metal in period 2 and the halogen in period 3.
Ans. Lithium, chlorine.
2. The noble gas having duplet arrangement of electrons.
Ans. He
3. The noble gas having an electronic configuration 2, 8, 8.
Ans. Ar
4. The number of electron shells in elements of period 3.
Ans. 3
5. The valency of elements in group 1[IA].
Ans. one
6. The metals present in period 3 and the non-metals present in period 2.
Ans.
Metals present in period 3 are Na, Mg and Al.
Non-metals present in period 2 are C, N, O and F.
7. The group whose elements have zero valency.
Ans. Inert gases i.e. Group 18
8. The non-metal in period 3 having a valency 1.
Ans. Cl
9. The formula of the hydroxide of the element having electronic configuration 2, 8, 2.
Ans. Mg (OH)2
10. The formula of the hydride of the halogen in period 3.
Ans. HCl
11. The formula of the sulphite of the element in period-3, group 1 [IA].
Ans. Na2S03
12. The element in period-3 which does not form an oxide.
Ans. Argon
13. The bonding [i.e. electrovalent or covalent] of the oxide of the element in period-3 group 16 [VIA].
Ans. Covalent
14. The character of the hydroxide of the element in period- 3 group 13 [IIIA].
Ans. Amphoteric
15. A light element in period-3 with a neutron/proton ratio around 1.
Ans.
Magnesium (Mg), Since the atomic mass of Mg is 24.305.
And it has 12 protons and 12.305 neutrons.
N/p ratio = 1.02 (which is nearest to 1)
16. The element with the least atomic size from carbon, nitrogen, boron and beryllium.
Ans. Nitrogen
17. The element, from the elements Li, Na, K, having the least number of electron shells.
Ans. Li
18. The element from the elements C, O, N, F, having the maximum nuclear charge.
Ans. F.
19. The element from the elements Be and Mg having a lower nuclear charge.
Ans. Be
20. The element from the elements fluorine and neon having a higher electron affinity.
Ans. F
21. The period and group to which the element ‘X’ with electronic configuration 2, 8, 8, 2 belongs.
Ans. Group 2, period 4
22. The more electro negative element from the elements Ar, S, Cl of period-3.
Ans. Cl
23. The element with the largest atomic size from the elements of period-1, 2 and 3.
Ans. Sodium (Na)
24. The element with the highest ionisation potential from the elements of period 1, 2 and 3.
Ans. Helium (He)
25. The element from the elements Li, Na, K which has maximum metallic character.
Ans. Potassium
26. The element with maximum non-metallic character from the elements of period-2.
Ans. Sulphur
27. The more non-metallic element from the elements S, P, Cl and Ar.
Ans. Cl (Chlorine)
28. The more non-metallic element from the elements ‘X’ and ‘Y’ having elec, config. 2,8,5 and 2, 8,6 respectively.
Ans. ‘Y’ 2, 8,6
29. The periodic property which relates to the amount of energy required to remove an electron from the outermost shell of an isolated gaseous atom.
Ans. Ionisation potential
30. The periodic property which refers to the character of element, which loses electron/s when supplied with energy.
Ans. Metallic property.
(b)
Fill in the blanks with the appropriate word/s in each case.
1
Periods are long [5, 6, 7] horizontal rows of elements in the periodic table and an element with three electron shells and two electrons in its valence shell belongs to period 3 [6, 3, 1] and group 2 [3, 6, 2].
2
Across a period the valence electrons increase by 1 while down a subgroup they remain same
3
Across a period, the electropositive character decreases and down a group the electronegative character decreases.
4
Elements at the extreme left of the modem periodic table are most reactive, while elements on the extreme right [group 18 (0)] are un reactive.
5
Elements of group 1 [IA] are strong reducing [oxidising / reducing] agents since they are electron donors [acceptors/ donors].
6
The element in group 17 [VILA] which is a liquid at room temperature is Br [F, Cl, Br, I]
7
Periodicity in properties is observed in elements after definite intervals due to similar Number of Valence electrons [electronic configuration, number of valence electrons, atomic numbers] of elements.
8
Across a period the nature of oxides and hydrides varies from basic to acidic while the strength of oxy-acids increases from left to right.
9
Nuclear charge of an atom is the positive charge on the nucleus of an atom, equivalent to the atomic number of an atom.
10
Atomic size of neon is more than the atomic size of fluorine.
11
Atomic size across a period decreases with increase in nuclear charge of the element. .
12
Ans. With increase in nuclear charge the nuclear attraction for outer electrons increases, hence ionisation potential increases.
13
Increase in nuclear charge of an atom decreases the tendency of the atom to lose electrons.
14
Elements with stable electronic configuration e.g. neon have an electron affinity value of 0.
15
An atom with a small atomic radii takes up electrons more readily than an atom with a large radii.
16
If combining atoms of a compound have nearly similar electronegativities the bond between them is covalent.
17
Elements with low electronegativity are usually metallic.
18
An atom is said to be a non-metal, if it gains one or more electrons.
19
Atoms with small atomic radii and high ionisation potential tend to gain electrons.
20
Element ‘X’ in period 3 has high electron affinity and electronegativity. It is likely to be a non-metal.
21
Element ‘B’ is likely to be more non-metallic in character than element‘A’.
22
Element ‘Z’ in sub-group 2[IIA] is below element ‘Y’ in the same sub-group. The element ‘Z’ will be expected to have higher atomic size and more metallic character than ‘Y’
23
Argon in period 3 is likely to have a larger atomic size than chlorine and its electron affinity value would be zero compared to chlorine.
24
Across a period- Atomic size and metallic character decreases while I.P., E.A., E.N. and non-metallic character increases and nuclear charge increases. Down a group-Atomic size and metallic character increases while I.P., E.A., E.N. and non- metallic character decreases and nuclear charge increases.
(c) Give reasons for the following:
Question 1.
In the same period or subgroup a gradual change in a particular property may be seen.
Answer:
In the same period or sub group a gradual change in particular property is due to the gradual change in electronic configuration in the arranged elements.
Question 2.
Atomic size of group 18 [0 group] elements is more than the atomic size of group 17 [VTIA] elements.
Answer:
In group 18 [0 group] inert gases, the outermost shell is completely filled resulting in force of repulsion increasing in atomic radii.
Question 3.
Ionisation potential increases with increase in nuclear charge of the elements.
Answer:
With increase in nuclear charge the nuclear attraction on the outer electrons increases. Hence the outer electrons are tightly held resulting in an increase in ionisation potential.
Question 4.
Electron affinity of noble gas elements is zero.
Answer:
Noble gas elements have completely filled outer-shell. Such electronic configurations are highly stable and as such noble gases find it difficult to accept electrons. Thus electron affinity of noble gas elements is zero.
Question 5.
Phosphorus, sulphur and chlorine are electronegative elements of the periodic table.
Answer:
The three non-metallic elements P, S and Cl are present at the end of period 3 in the modern periodic table. Along a period nuclear charge increases while atomic size decreases. Both of these factors increase the electronegativity. Thus these elements have highest electronegativity.
Question 6.
Sulphur is placed in group 16 [VIA], chlorine in group 17[VIIA] but argon in group 18 [0 group] of the pericdic table.
Answer:
Electronic configurations of S, Cl and Ar are:
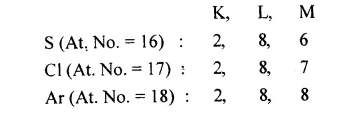
With 6 electrons in the outermost or valence shell, S is placed in Group 16. In the same way Cl with 7 electrons in the valence shell is placed in Group 17 and Ar with 8 electrons in the valence shell is placed in Group 18.
Question 7.
Fluorine is the most electronegative element of the periodic table.
Answer:
Except for noble gases, electronegativity increases along a period from left to right and decreases down a group from top to bottom. Therefore, fluorine present at the upper right hand comer of the modem long form of the periodic table has highest electronegativity.
Question 8.
Atoms with large atomic radii and low ionisation potential are more metallic in nature.
Answer:
Metals are electropositive in nature i.e., they can lose one or more electrons easily. Atoms with large atomic radii and low ionisation potential can easily lose one or more electrons as the nuclear pull on the outer electrons is less. As such, these atoms are more metallic in nature.
Question 9.
A decrease in ionisation potential of an element leads to a decrease in non-metallic character of the element.
Answer:
Due to decrease in ionisation potential tendency to lose electrons increases. This results in an increase in metallic character and a corresponding decrease in non-metallic character. Thus elements with lower ionisation potential are less non-metallic in nature.
Question 10.
Atomic size decreases across a period but increases down a group of the periodic table.
Answer:
As we move along a period from left to right in the periodic table, number of shells remains the same while nuclear charge increases by one at each step. Due to increase in nuclear charge electrons in the outermost shell are attracted with increasing force resulting in a gradual decrease in atomic size along a period.
As we move down a group from top to broom, number of shells as well as nuclear charge increases. However, this effect of increase in the number of shells dominates over increase in nuclear charge. As such, atomic size increases down a group in the periodic table.
–: End of Periodic Table :–
Return to New Simplified Dalal Chemistry for ICSE Class-10
Thanks
Please Share with Your Friends