Physical And Chemical Changes Class-8th Goyal Brothers ICSE Chemistry Solutions Ch-2 Unit 1 . We Provide Step by Step Answers of Objectives, True and False, Incorrect and Correct , Definitions , Match the followings and Short/Long Question Type answers of Chapter-2, Unit 1, Physical And Chemical Changes. Visit official Website CISCE for detail information about ICSE Board Class-8
Physical And Chemical Changes Class-8th Goyal Brothers ICSE Chemistry Solutions Ch-2
| Board | ICSE |
| Class | 8th |
| Subject | Chemistry |
| Book Name | Goyal Brothers |
| Chapter-2 | Physical And Chemical Changes |
| Unit-1 | Physical And Chemical Changes |
| Topic | Solution of exercise questions |
| Session | 2023-24 |
OBJECTIVE TYPE QUESTIONS
Physical And Chemical Changes Class-8th Goyal Brothers ICSE Chemistry Solutions Ch-2
Que: A. Fill in the blank spaces by choosing the correct words from the list given below :
List : liquid, hydrogen sulphide , exothermic, yellow, dirty green
- When quick lime is placed in water an …exothermic… reaction takes and the reaction mixture gets very hot.
- When red hot coke (solid state), reacts with sulphur vapour the product, carbon disulphide is in …liquid… state.
- Ferrous sulphate solution reacts with sodium hydroxide solution to form a …hydrogen sulphide.. precipitate of ferrous hydroxide.
- Lead nitrate crystals on heating strongly leave behind a solid residue, which is in …yellow… colour.
- When copper sulphide is treated hydrochloric acid it gives off a …dirty green…which has a foul smell.
Que : B. Statements given below are incorrect. Write the correct statements.
Questions 1: Lead nitrate crystals on strong heating decompose to form lead dioxide, nitrogen dioxide and oxygen gas.
Answer 1: Lead nitrate crystals on strong heating decompose to form lead monoxide, nitrogen dioxide and oxygen gas.
Questions 2: The reaction between magnesium and oxygen is an endothermic reaction.
Answer 2: The reaction between magnesium and oxygen is an exothermic reaction.
Questions 3: The decomposition of ammonium chloride into ammonia and oxygen is an exothermic reaction.
Answer 3: The decomposition of ammonium chloride into ammonia and oxygen is an endothermic reaction.
Questions 4: Copper carbonate on strong heating leaves behind a residue which is green in colour.
Answer 4: Copper carbonate on strong heating leaves behind a residue which is black in colour.
Questions 5: Ammonia gas and HCI gas react to form gaseous ammonium chloride.
Answer 5: Ammonia gas and HCI gas react to form solid ammonium chloride.
Que: C. Match the statements in Column A, with those in Column B.
| Column A | Column B |
| 1. A chemical reaction which proceeds with the evolution of heat. | (a) Chemical change |
| 2. A chemical reaction which proceeds with the absorption of heat. | (b) Exothermic reaction |
| 3. Name of a change brought about by the decomposition of fruit juice. | (c) Oxygen |
| 4. Name of change brought about when current passes through an electric bulb. | (d) Endothermic reaction |
| 5. A gas given out when sodium nitrate crystals are heated strongly. | (e) Physical change |
Answer :
| Column A | Column B |
| 1. A chemical reaction which proceeds with the evolution of heat. | (a)Exothermic reaction |
| 2. A chemical reaction which proceeds with the absorption of heat. | (b)Endothermic reaction |
| 3. Name of a change brought about by the decomposition of fruit juice. | (c)Chemical change |
| 4. Name of change brought about when current passes through an electric bulb. | (d)Physical change |
| 5. A gas given out when sodium nitrate crystals are heated strongly. | (e)Oxygen |
Que :D. Write ‘true’ or ‘false’ for the following statements :
| Statement | True/False |
| 1. Gold on hammering can form a thin sheet of gold. | |
| 2. Burning of hydrogen and oxygen to form water is an example of physical change. | |
| 3. Hydrogen gas react with chlorine gas to form liquid hydrochloric acid. | |
| 4. Red lead oxide on strong heating gives off oxygen gas. | |
| 5. Lead nitrate solution on mixing with potassium iodide solution forms green precipitate of lead iodide |
Answer :
| Statement | True/False |
| 1. Gold on hammering can form a thin sheet of gold. | T |
| 2. Burning of hydrogen and oxygen to form water is an example of physical change. | F |
| 3. Hydrogen gas react with chlorine gas to form liquid hydrochloric acid. | F |
| 4. Red lead oxide on strong heating gives off oxygen gas. | T |
| 5. Lead nitrate solution on mixing with potassium iodide solution forms green precipitate of lead iodide | F |
Que : E. Tick (√) the most appropriate answer :
Questions 1: The reaction between magnesium and oxygen is :
(a) an endothermic reaction
(b) an exothermic reaction
(c) a catalysed reaction
(d) a reversible reaction
Answer 1: option (b) an exothermic reaction is correct.
Questions 2: The decomposition of sodium bicarbonate on heating is :
(a) an endothermic reaction
(b) a reversible reaction
(c) a catalysed reaction
(d) an exothermic reaction
Answer 2: option (a) an endothermic reaction is correct.
Questions 3: When lead nitrate crystals are strongly heated, the colour of one of the gas evolved is :
(a) greenish yellow
(b) pink
(c) reddish brown
(d) light grey
Answer 3: option (c) reddish brown is correct.
Questions 4: When ammonia gas reacts with hydrochloric acid gas, the ammonium chloride is formed which is in :
(a) liquid state
(b) solid state
(c) gaseous state
(d) partly solid and partly gaseous state
Answer 4: option (c) gaseous state is correct.
Questions 5: When silver nitrate solution is mixed with sodium chloride solution, a precipitate of silver chloride is formed, which is of :
(a) silvery colour
(b) yellow colour
(c) grey colour
(d) white colour
Answer 5: option (d) white colour is correct.
STUDY QUESTIONS
Physical And Chemical Changes Class-8th Goyal Brothers ICSE Chemistry Solutions Ch-2
Question 1: Differentiate between :
(a) Slow and Fast changes
| Slow changes | Fast changes |
|
|
(b) Desirable and undesirable change
| Desirable change | Undesirable change |
|
|
|
|
(c) Periodic and non-periodic changes
| Periodic changes | Non-periodic changes |
| Those events that repeat themselves after a fixed period of time and their occurrence can be predicted are called periodic changes. | The events that do not repeat themselves after regular intervals of time are called nonperiodic changes. |
| Examples of periodic change are the occurrence of day and night. | The burning of the matchstick is a non-periodic change. |
(d) Reversible and irreversible changes
| Irreversible change | Reversible change | |
| A change is called irreversible if it cannot be changed back again. | A reversible change is a change that can be reversed. | |
| For example, you cannot change a cake back into its ingredients again. | For example, melted chocolate can be changed back into solid chocolate by cooling. | |
| Irreversible changes are permanent. They cannot be undone. For example, combustion cannot be undone. |
Reversible changes are not permanent. They can be undone. For example, we can freeze orange juice to make ice lollies. The ice lollies can be changed back into orange juice by heating. |
|
| In an irreversible reaction, the reactants react to form the products, which cannot revert back into reactants | In reversible reactions, as the reactants react with other reactants to form products, the products are reacting with other products to form reactants. |
Question 2: What do you understand by the following terms ?
Answer 2: (a) Physical change : A change in matter which does not alter the chemical properties of the matter. For example, if we carve a piece of wood into a baseball bat, it will still burn in a fire and float on water.
(b) Chemical change :- A chemical change is a change of materials into another, new materials with different properties and one or more than one new substances are formed.
(c) Chemical reaction – A chemical reaction is a process in which one or more substances, also called reactants, are converted to one or more different substances, known as products. Substances are either chemical elements or compounds.
Question 3: Give two examples which illustrate the evolution of a gas during a chemical reaction.
Answer 3: The evolution of a gas during a chemical reaction two examples –
- electricity is passed through Sodium chloride solution, Sodium chloride decomposes into Sodium and, Chlorine gas is evolved.
- When Zinc is placed in Sulphuric acid, Zinc is more reactive than Hydrogen displaces it from the acid, and forms zinc sulfate and hydrogen gas.
Question 4:- . Give two examples which illustrate the change in the colour of reactants during a chemical reaction.
Answer 4: The change in the colour of reactants during a chemical reaction two examples –
- The type of reaction where one atom or a set of atoms is replaced with another atom within the same molecule is called a displacement reaction.
- For example, when silver nitrate is reacted with copper, the copper displaces the silver from the silver nitrate solution and forms a copper nitrate solution.
Question 5: Illustrate the formation of precipitate during a chemical reaction.
Answer 5: One of the best examples of precipitation reactions is the chemical reaction between potassium chloride and silver nitrate, in which solid silver chloride is precipitated out. This is the insoluble salt formed as a product of the precipitation reaction.
Question 6: Define an exothermic reaction. Support your answer with two examples.
Answer 6: The chemical reaction in which the heat is released is known as an Exothermic reaction. Examples are the burning of a candle and the reaction of a strong acid with water.
Question 7: Define an endothermic reaction. Support your answer with one example.
Answer 7: The chemical reaction in which the heat is absorbed is known as an Endothermic reaction. Examples are melting ice cubes and melting solid salts.
Question 8: Give two examples to show a change of state during a chemical reaction.
Answer 8: Chemical reaction – A chemical reaction is the change of a substance into a new one that has a different chemical identity.
- Some chemical reactions are observed by the state change of the reactants.
- Below are two examples of it.
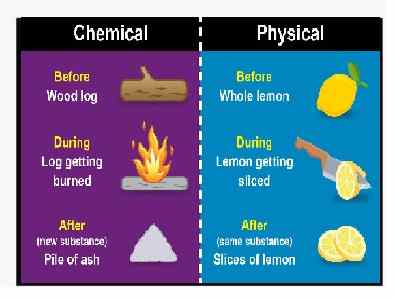
Question 9: Differentiate between physical and chemical changes.
| Physical change | Chemical change |
| Generally, physical changes do not involve the production of energy. | Chemical changes usually involve the production of energy (which can be in the form of heat, light, sound, etc.) |
| In a physical change, no new substance is formed. | A chemical change is always accompanied by one or more new substance(s). |
| Physical change is easily reversible i.e original substance can be recovered. | Chemical changes are irreversible i.e. original substance cannot be recovered. |
— : end of Physical And Chemical Changes Class-8th Goyal Brothers Answers :–
Return to- ICSE Class -8 Goyal Brothers Chemistry Solutions
Thanks
Please share with yours friends if you find it helpful.


