States of Matter ICSE Class-8th Goyal Brothers Prakashan Physics Solutions Chapter-1. We Provide Step by Step Answers of Objective, True False , Fill in the blanks, Match the following , Short/Long Answer Type of Exercise-1 Matter, Unit-1 Visit official Website CISCE for detail information about ICSE Board Class-8.
States of Matter ICSE Class-8th Goyal Brothers Physics Solutions Ch-1 (unit-1)
On The Basis of Kinetic theory
| Board | ICSE |
| Class | 8th |
| Subject | Physics |
| Book Name | Goyal Brothers |
| Chapter-1 | Matter |
| Unit-1 | States of Matter On The Basis of Kinetic theory |
| Topic | Solution of exercise questions |
| Session | 2023-24 |
OBJECTIVE TYPE QUESTIONS
A. Fill in the blanks spaces by choosing the correct words from the list given below :
- Kinetic energy of the molecules _increase_ with an increase in temperature .
- With an increase in the intermolecular _space_, the intermolecular forces decrease.
- The phenomenon due to which the exposed surface of a liquid behaves like a stretched membrane is called _surface tension_.
- Mercury forms small spherical droplets, because it has no force of _adhesion_ with the surface of glass.
- The meniscus formed by water in a test tube is of _concave_ shape.
B. Statement given below are incorrect. write the correct correct statement:
1. A molecule is the smallest unit of matter which may or may not have an independent existence, but always takes part in a chemical reaction.
Correct Statement: An atom is the smallest unit of matter which may or may not have an independent existence, but always takes part in a chemical reaction
2. Meniscus is formed in case of confined liquids due to the force of surface tension.
Correct Statement: Meniscus is formed in case of confined liquids due to the force of cohesion.
3. The pressure of an enclosed gas increases with the increase in volume.
Correct Statement: The pressure of an enclosed gas increases with the decrease in volume.
4. The molecules of a gas have very small intermolecular spaces.
Correct Statement: The molecules of a gas have very large intermolecular spaces.
5. Solids have definite mass, but no definite volume.
Correct Statement: Solids have definite mass and definite volume.
C. Write ‘True’ or ‘False’ for the following statements :
1. With the decrease in intermolecular spaces, the intermolecular forces decrease. [F]
2. Solids get heated by conduction only.[T]
3. With the decrease in the volume of a gas its pressure increases. [T]
4. Mercury in a test tube forms concave meniscus. [F]
5. The position of molecules in a liquid continuously change. [T]
D. Tick (√) the most appropriate answer.
1. The intermolecular spaces in case of liquids are :
(a) less than the solids.
(b) more than the gases.
(c) more than the solids and less than the gases
(d) more than the solids and gases
Ans: (c) more than the solids and less than the gases
2. The kinetic energy of molecules of a gas increases with :
(a) fall in temperature
(b) rise in temperature
(c) decrease in pressure
(d) increase in pressure
Ans: (b) rise in temperature
3. Heat is transmitted in liquids by the process of :
(a) Conduction
(b) Convection
(c) Radiation
(d) both Conduction and Convection
Ans: (b) Convection
4. The top surface of a liquid behaves like stretched membrane because :
(a) The molecules below the top surface exert upward pressure.
(b) The molecules are attracted more to the sides of the containing vessel.
(c) The molecules below the top surface attract the molecules above.
(d) none of these
Ans: (b) The molecules are attracted more to the sides of the containing vessel.
5. The meniscus formed by mercury in a test tube is :
(a) Convex
(b) Concave
(c) no meniscus is formed
(d) none of these
Ans: (a) Convex
IV. Match the statement in Column A with those in Column B:
Column A |
Column B |
1. The phenomenon due to which the top surface of a liquid behaves like stretched membrane. |
1. Gas pressure |
2. A substance which cannot be subdivided into two or more simpler substances by any chemical means. |
2. intermolecular force |
3. The force of attraction between the molecules of a given substance. |
3. Adhesive force |
4. The force exerted per unit area by the molecules of a gas on the sides of containing vessel. |
4. Surface tension |
5. The force due to which ink sticks to a piece of paper. |
5. Element |
Answer:
| Column A | Column B |
| 1. The phenomenon due to which the top surface of a liquid behaves like stretched membrane. | 4. Surface tension |
| 2. A substance which cannot be subdivided into two or more simpler substances by any chemical means. | 5. Element |
| 3. The force of attraction between the molecules of a given substance. | 2. intermolecular force |
| 4. The force exerted per unit area by the molecules of a gas on the sides of containing vessel. | 1. Gas pressure |
| 5. The force due to which ink sticks to a piece of paper. | 3. Adhesive force |
STUDY QUESTIONS
Question 1. Define the terms :
(a)Element
(b)Atom
(c)Molecule
Answer:
(a) Element: An element is a pure substance that cannot be broken down by any physical or chemical means into simpler substances. An element is made up of the same kind of atoms.
(b) Atom: An atom is a particle of matter that uniquely defines a chemical element. An atom consists of a central nucleus that is surrounded by one or more negatively charged electrons.
(c) Molecule: Molecules are made up of one or more atoms. If they contain more than one atom, the atoms can be the same (an oxygen molecule has two oxygen atoms) or different (a water molecule has two hydrogen atoms and one oxygen atom). Biological molecules, such as proteins and DNA, can be made up of many thousands of atoms.
Question 2. State five important assumptions of the kinetic theory of matter.
Answer: Five important assumptions of the kinetic theory of matter are :-
(1) When molecules collide with each other, no energy is gained or lost.
(2) Have negligible volume
(3) Have negligible intermolecular forces
(4) Undergo perfectly elastic collisions
(5) Have an average kinetic energy proportional to the ideal gas’s absolute temperature.
Question 3. Explain the following on the basis of kinetic theory of matter.
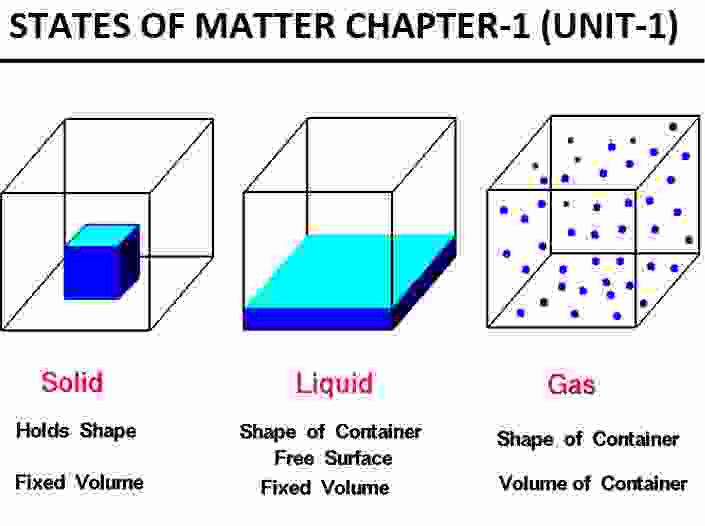
(a) Solids have a definite volume and definite shape
Answer: If particles do not have enough kinetic energy to overcome the force of attraction between them, matter exists as a solid. The particles are packed closely together and held rigidly in place. All they can do is vibrate. This explains why solids have a fixed volume and a fixed shape.
(b) Liquids have a definite volume, but no definite shape
Answer: This is due to the fact that the intermolecular force of attraction between molecules in liquids is lower than in solids, and the liquid molecules are loosely packed. Because the particles in a liquid are in close proximity, they have a defined volume but not a definite shape.
(c) Gases have no definite volume and no definite shape
Answer: This is due to the fact that the intermolecular force of attraction between molecules in liquids is lower than in solids, and the liquid molecules are loosely packed. Because the particles in a liquid are in close proximity, they have a defined volume but not a definite shape
Question 4.
(a) What do you understand by the term surface tension?
Answer: Surface tension is a property that allows the surface of a liquid to behave as a stretched membrane. The term surface tension is used when a liquid surface is in contact with gas or air. It is a contact force.
(b) Name the force responsible for surface tension in liquids.
Answer: The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension
Question 5. Explain the following :
(a) A very small amount of mercury on placing over a clean glass plate forms a tiny spherical ball.
Answer: Mercury remains spherical on a glass plate due to greater cohesive force between mercury molecules than adhesive force between mercury and glass plate.
(b) A very small amount of water on placing over a clean glass plate forms an oval shaped drop.
Answer: A very small amount of water on placing over a clean glass plate forms an oval shaped drop because the force of attraction between the molecules of water, that is, the force of cohesion is far less than the force of adhesion between the molecules of water and the glass plate and hence it takes an oval shape.
Question 6.
(a) What do you understand by the term meniscus?
Answer: It is observed that when a free surface of a liquid comes in contact with a solid, it shows a curvy shape near the point of contact. Here an inward depression or an outward bulge takes place. This is called meniscus of a liquid
(b) Name the kind of meniscus formed
-
in case of water
-
in case of mercury
Answer: The kind of meniscus formed are:-
- in case of water: A concave meniscus is formed.
- in case of mercury: A concave meniscus is formed.
Question 7. On the basis of kinetic model, explain how the liquids exert pressure.
Answer: All the liquids exert pressure on the base or bottom and walls of their container. All the liquid have weight. When we pour liquid into a vessel, then the weight of liquid pushes down on the base of the vessel producing a pressure. The liquid exerts pressure on the base of the vessel
Question 8. On the basis of kinetic model explain how the gases exert pressure.
Answer: The theory further explains that gas pressure is generated when particles collide with each other or collide with the wall of a container. According to the Kinetic theory of gas’s assumption, gas molecules collide with the wall and change their momentum, applying force to the wall and creating pressure
Question 9. Explain the heating of an iron rod by conduction on the basis of kinetic model.
Answer: conduction is the process when heat spread through a medium. as there is kinetic energy between the atoms of matter so when the iron rod is heat up the whole rod get hot after a time due to the kinetic intermolecular forces the hit is passed through whole rod
Question 10. Liquids and gases gets heated by convection. Explain the convection on the basis of kinetic model.
Answer: Heat convection on kinetic model of matter
On expansion they become lighter than the rest of the molecules of the substance and so they rise upwards taking the heat energy along with them. This results in the generation of convectional currents taking the warmer molecules up while sinking the colder molecules down.
— : end of States of Matter Class-8th Goyal Brothers :–-
Return to- ICSE Class -8 Goyal Brothers Physics Solutions
Thanks
Please share with your friends if you find it useful